Mosaic type virus-like particle DNA vaccine
A DNA vaccine and virus-like technology, applied in the field of DNA vaccines, can solve the problems of complex preparation and purification process and high cost, and achieve the effect of protecting against pathogen infection, simple preparation and improved ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, the preparation of chimeric virus-like particle DNA vaccine
[0039] 1. Construction of the recombinant expression vector proVAX-ABC containing the gene encoding the chimeric protein HBc-VP1
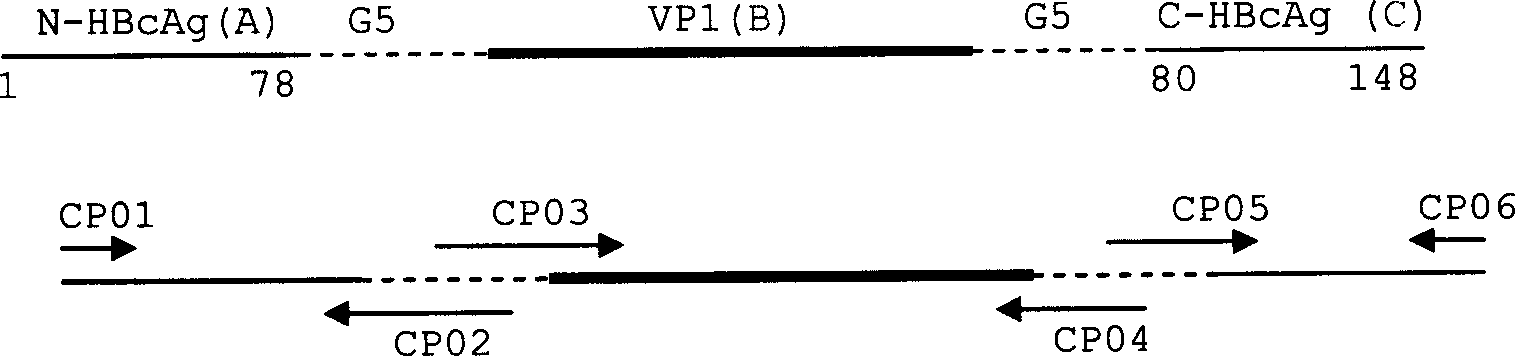
[0040] In order to enable the foot-and-mouth disease virus VP1 gene to be effectively expressed in the human hepatitis B virus core antigen and to be displayed outside the core antigen, the foot-and-mouth disease virus VP1 gene fragment was cloned into the 78th amino acid and the 80th amino acid from the amino terminal of the human hepatitis B virus core antigen. between the codons of the amino acids to facilitate the display and presentation of the antigen. The specific method is as follows:
[0041] (1) Primer design: according to the sequences of human hepatitis B virus core antigen gene and foot-and-mouth disease virus VP1 gene, and according to figure 1 3 sets of primers were designed according to the cloning ideas in . The primer sequences are
[0042] CP01...
Embodiment 2
[0056] Example 2, Animal experiment of chimeric virus-like particle DNA vaccine proVAX-ABC
[0057] 1. Mouse experiment
[0058] 4-6 weeks old female C57BL / 6 mice (purchased from the Institute of Experimental Animals, Chinese Academy of Medical Sciences), divided into 4 groups, 6 mice in each group, were immune to proVAX-VP1 (100 μg / mouse / time), proVAX-ABC (100μg / one / time), proVAX (100μg / one / time) and its PBS control group (100μl / one / time). Each mouse was first immunized on day 0 and boosted once on day 14. Mouse serum was collected 14, 28, 42, 56, and 70 days after the first immunization, and stored at -20°C for future use.
[0059] (1) ELISA detection of IgG antibody levels in proVAX-VP1 immunized mice
[0060] Coat 96-well ELISA plate with 1 μg / ml 146S antigen (remove the mineral oil in the bovine foot-and-mouth disease O-type inactivated vaccine (purchased from Inner Mongolia Jinyu Biopharmaceutical Factory) to obtain 146S antigen), 100 μl / well, overnight at 4°C; PBST ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com