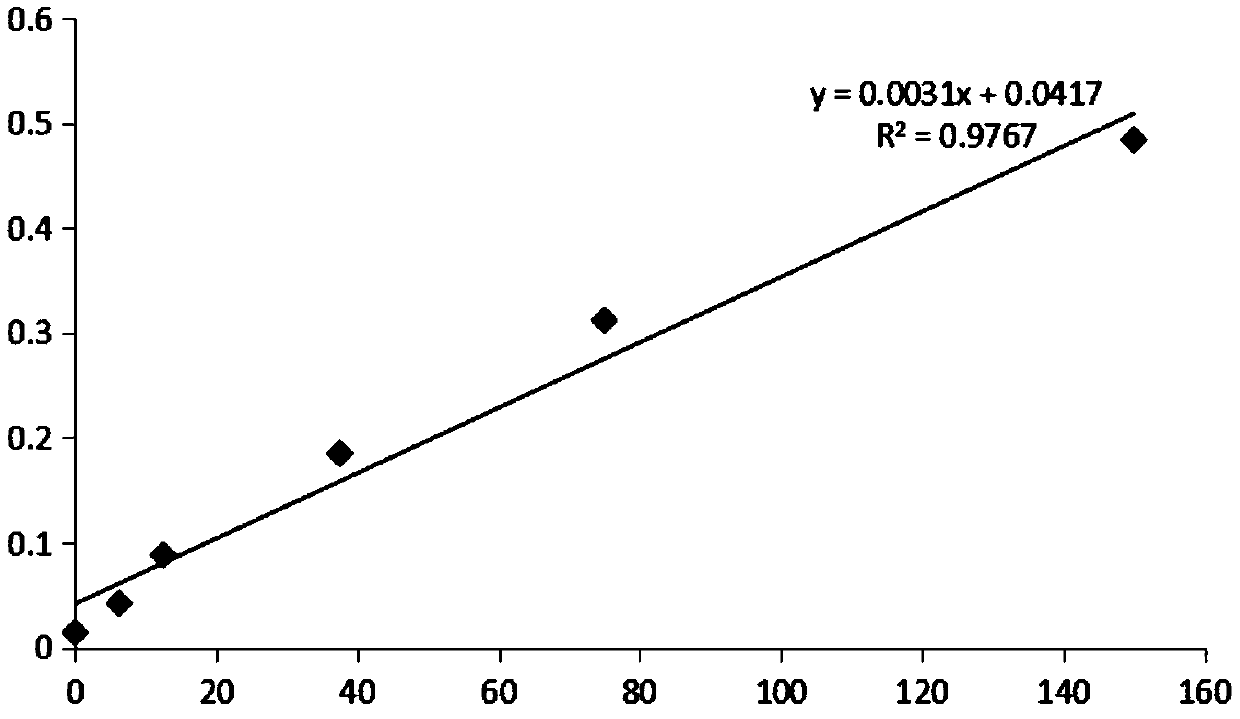
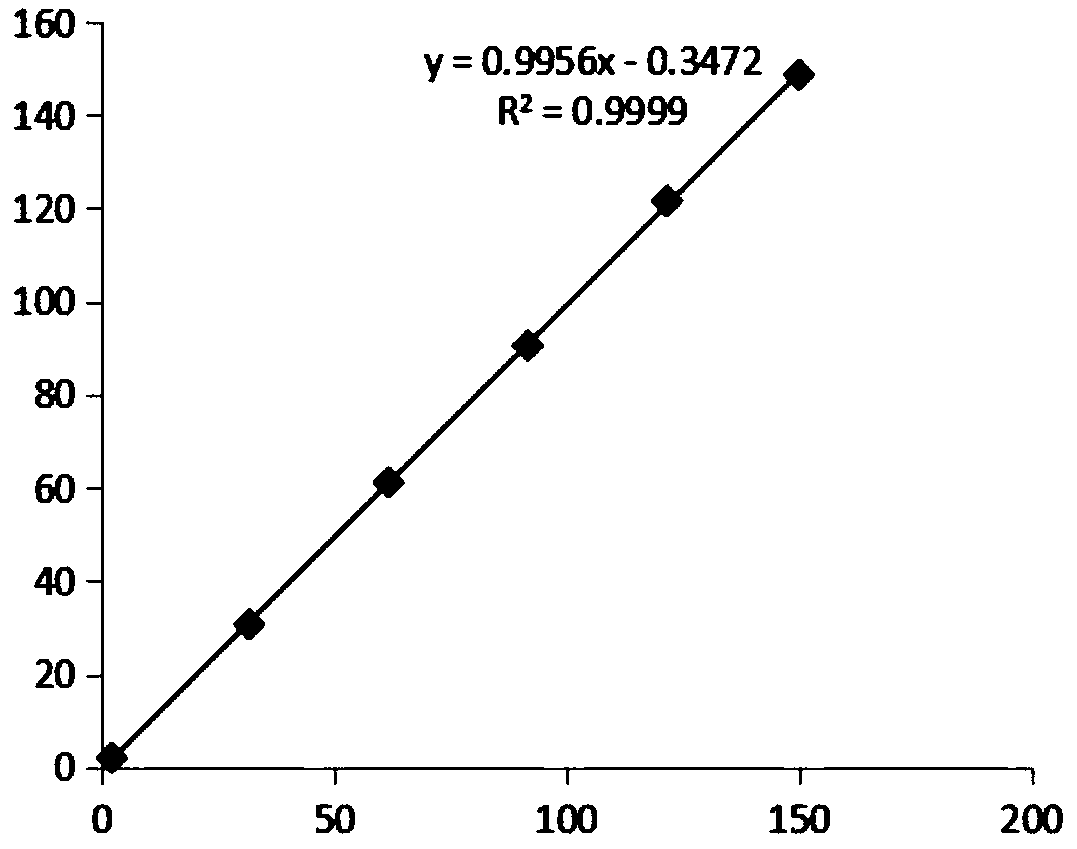
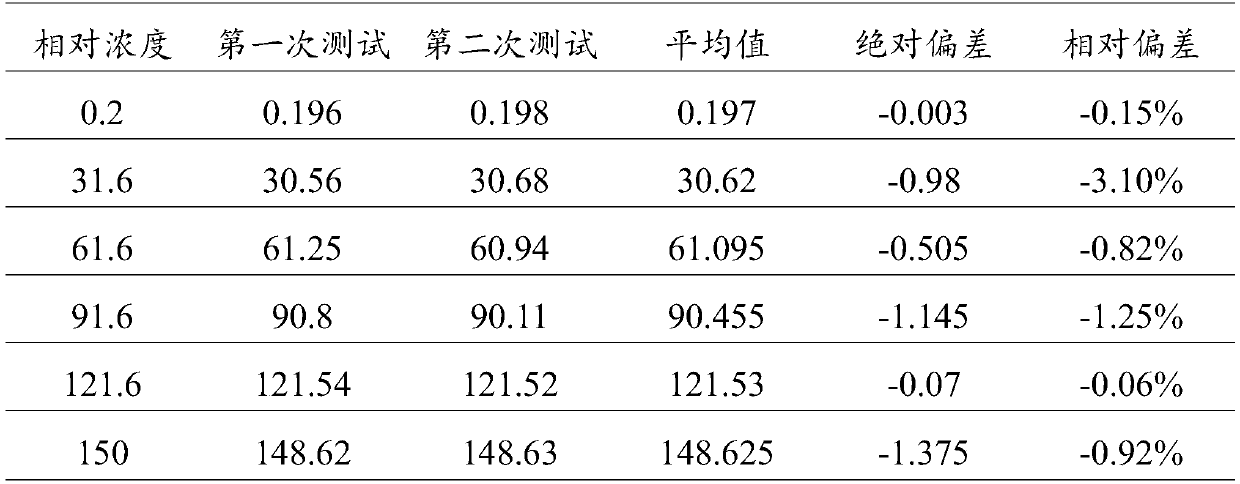
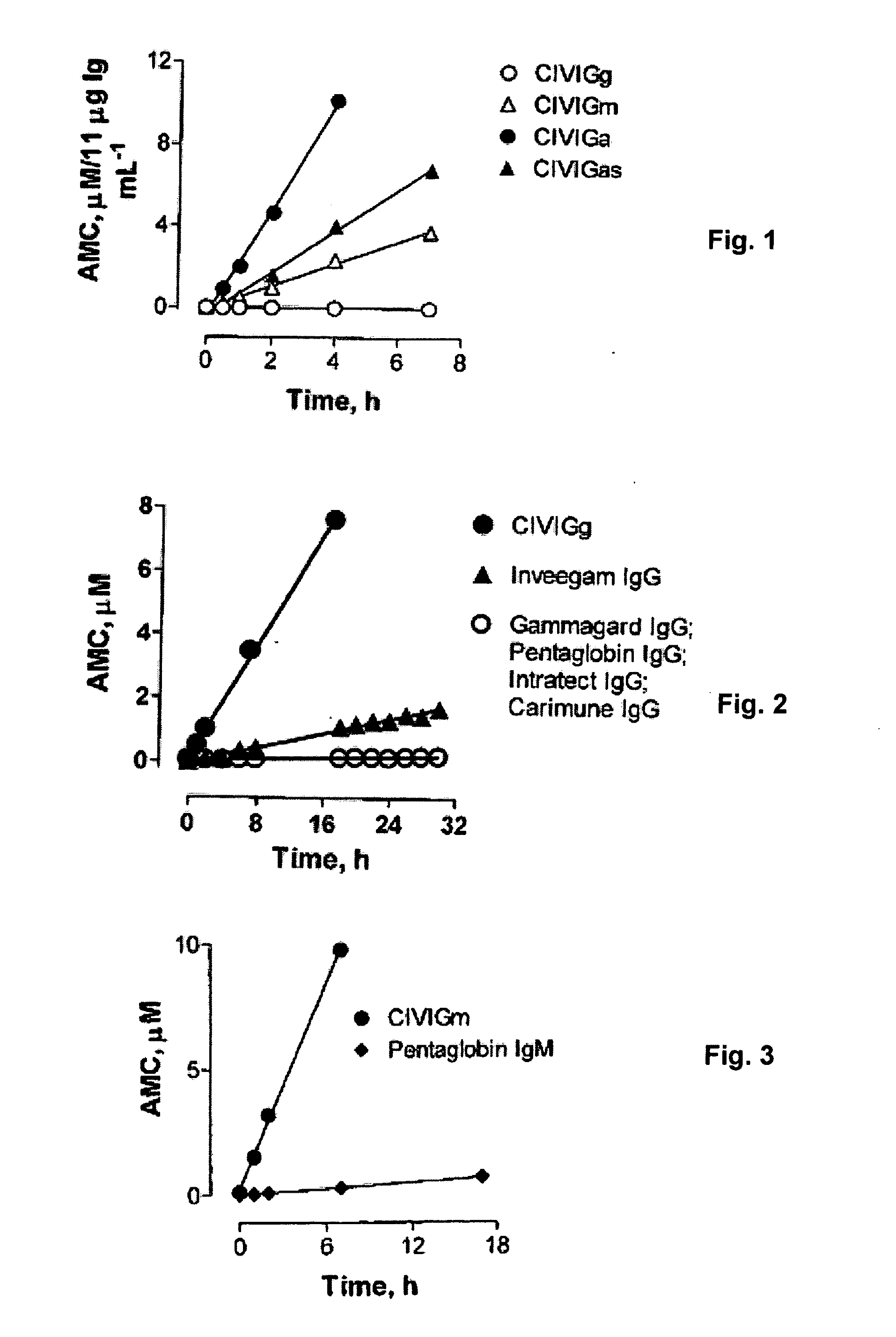
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
72 results about "Human immunoglobulins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Human immunoglobulins can be divided into 5 Immunoglobulin classes: IgM, IgD, IgA, IgG, IgE. IgA is composed of two (IgAl, IgA2) and IgG of four subclasses (IgG1, IgG2, IgG3, IgG4).
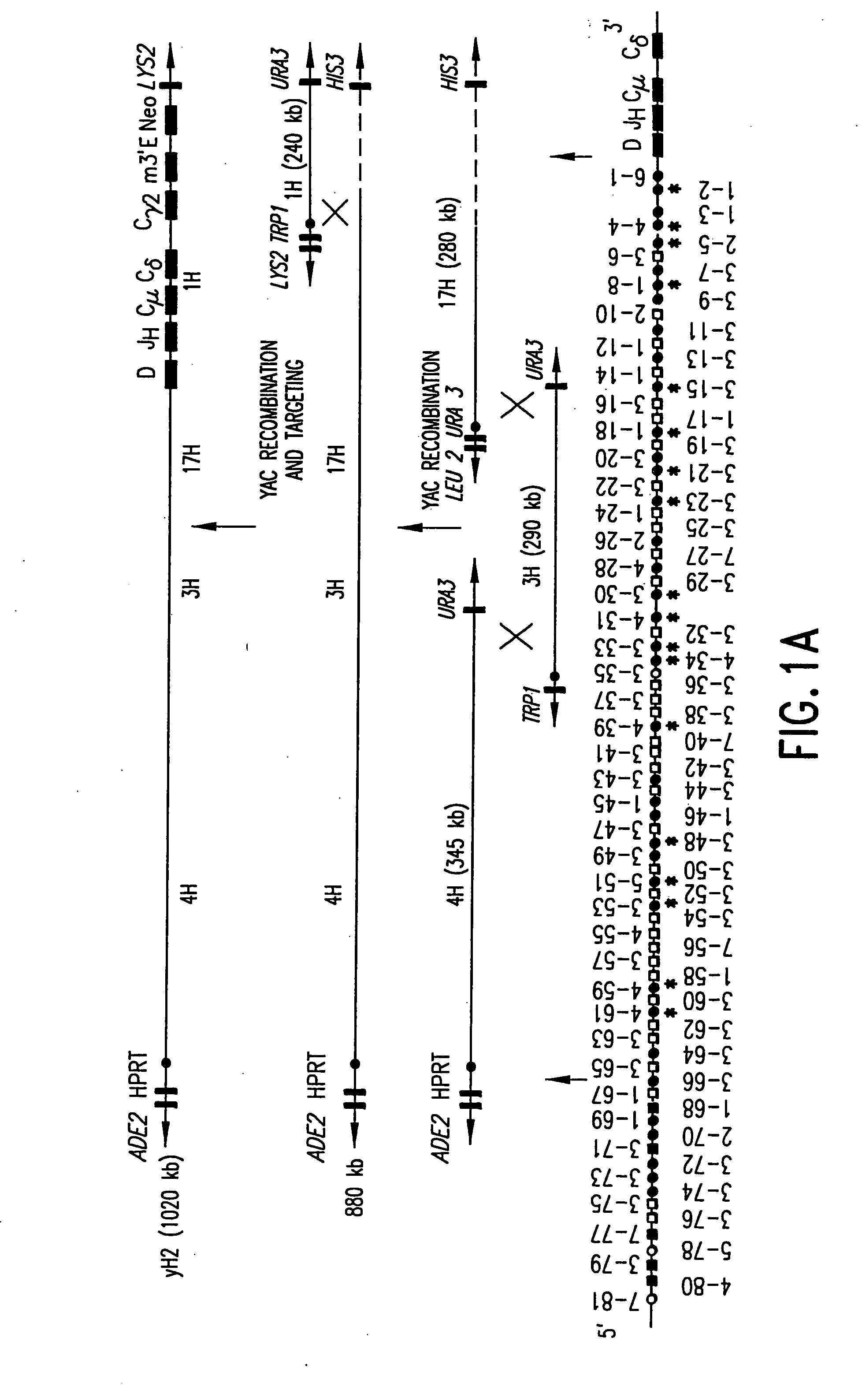
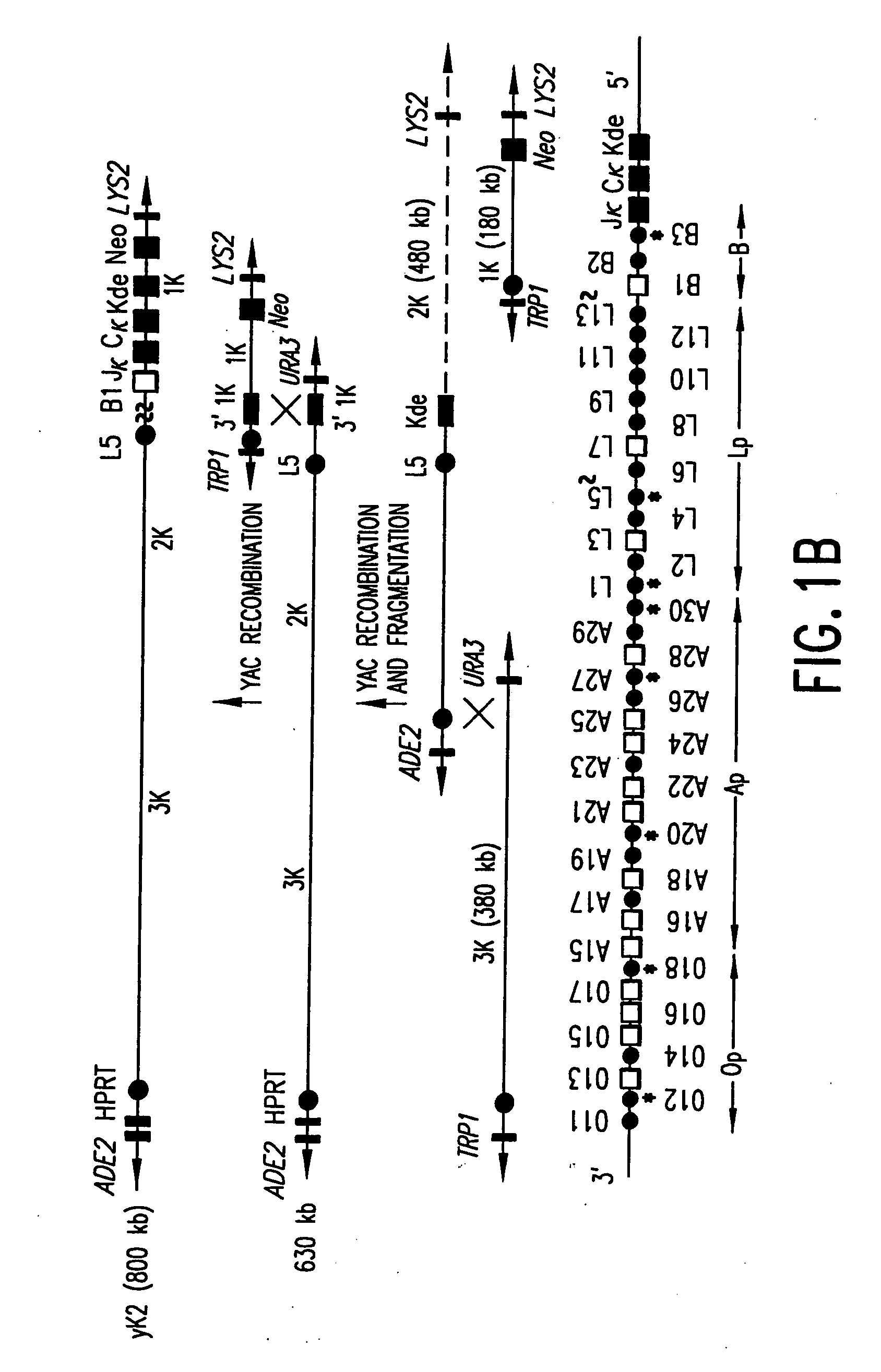
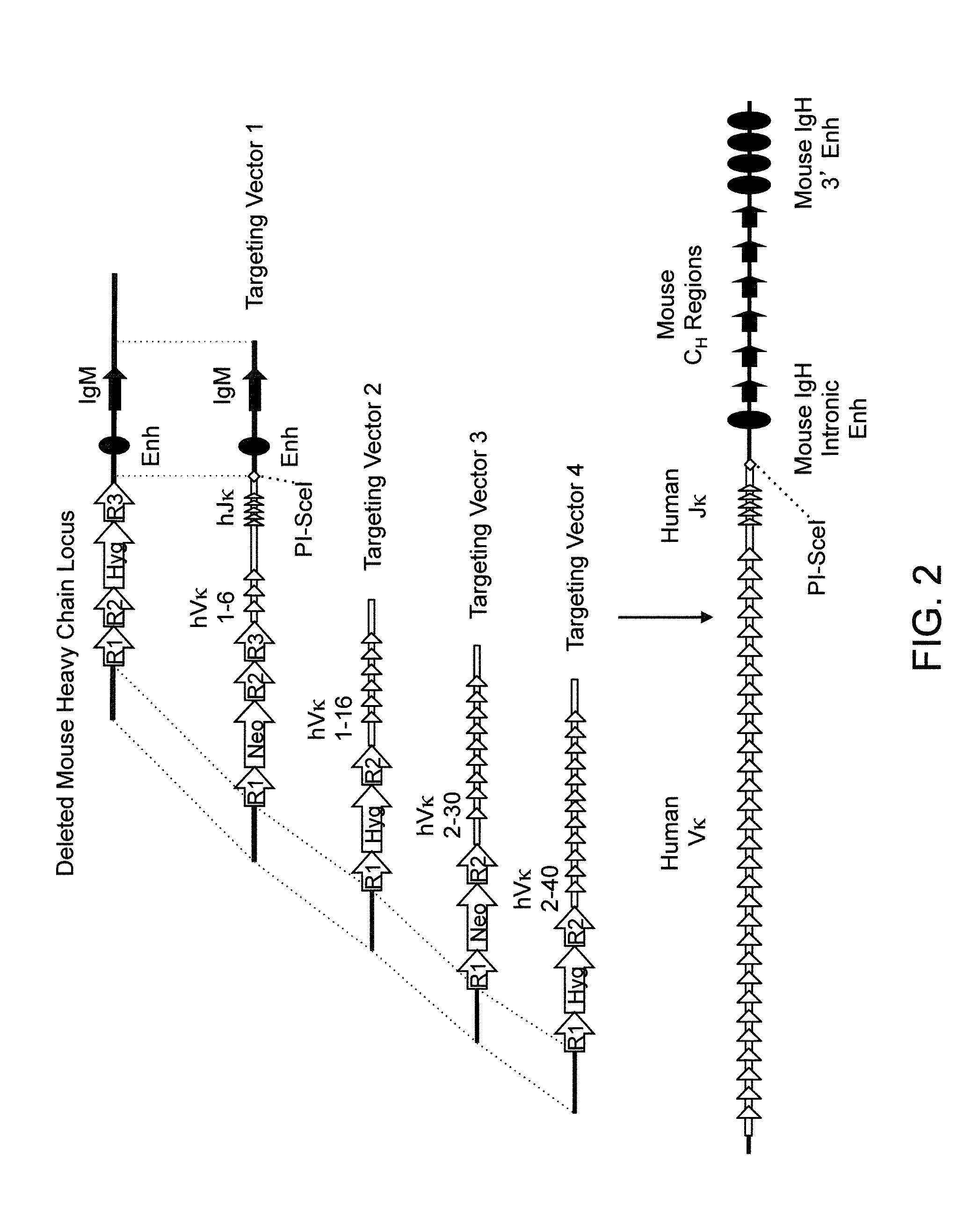
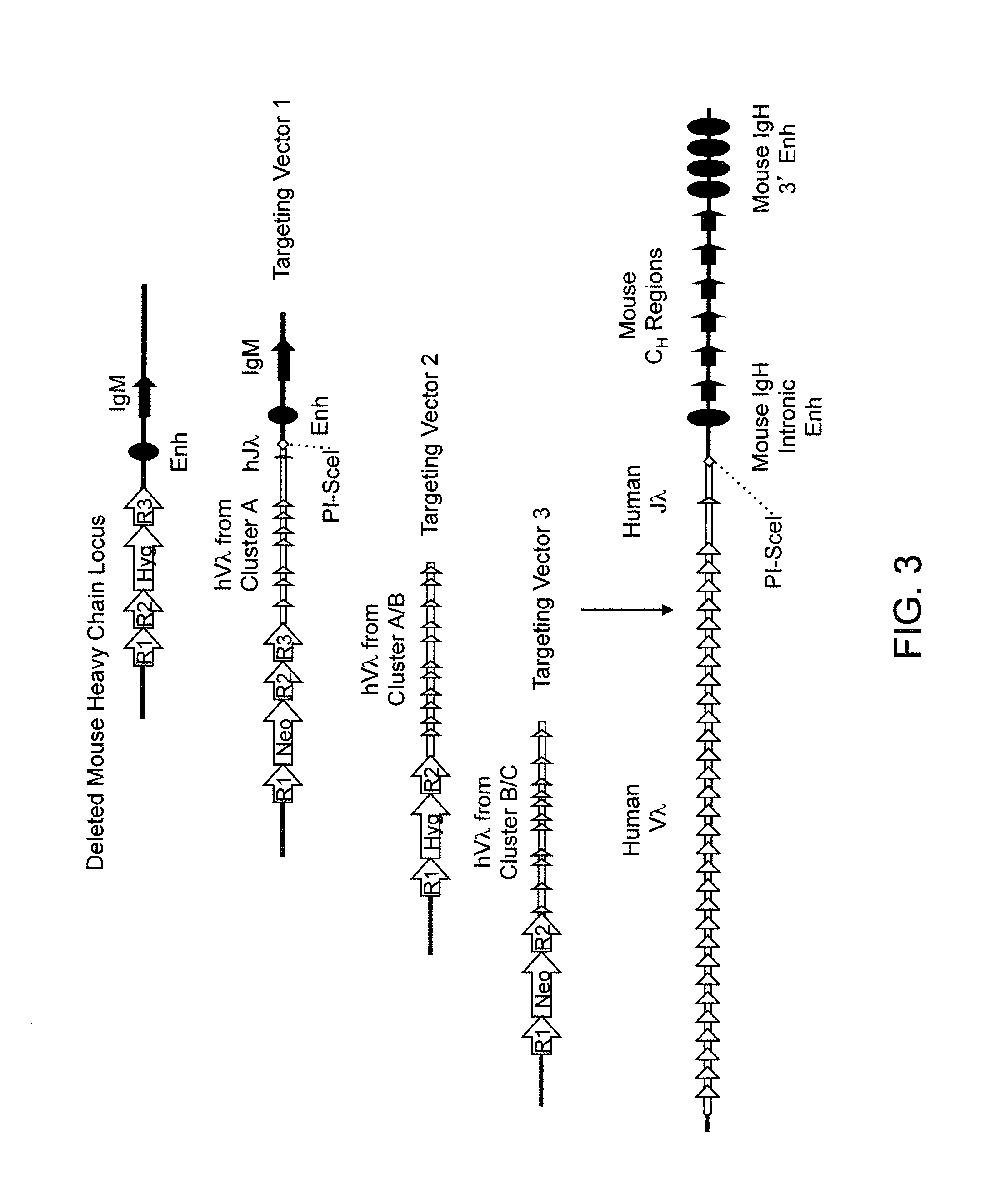
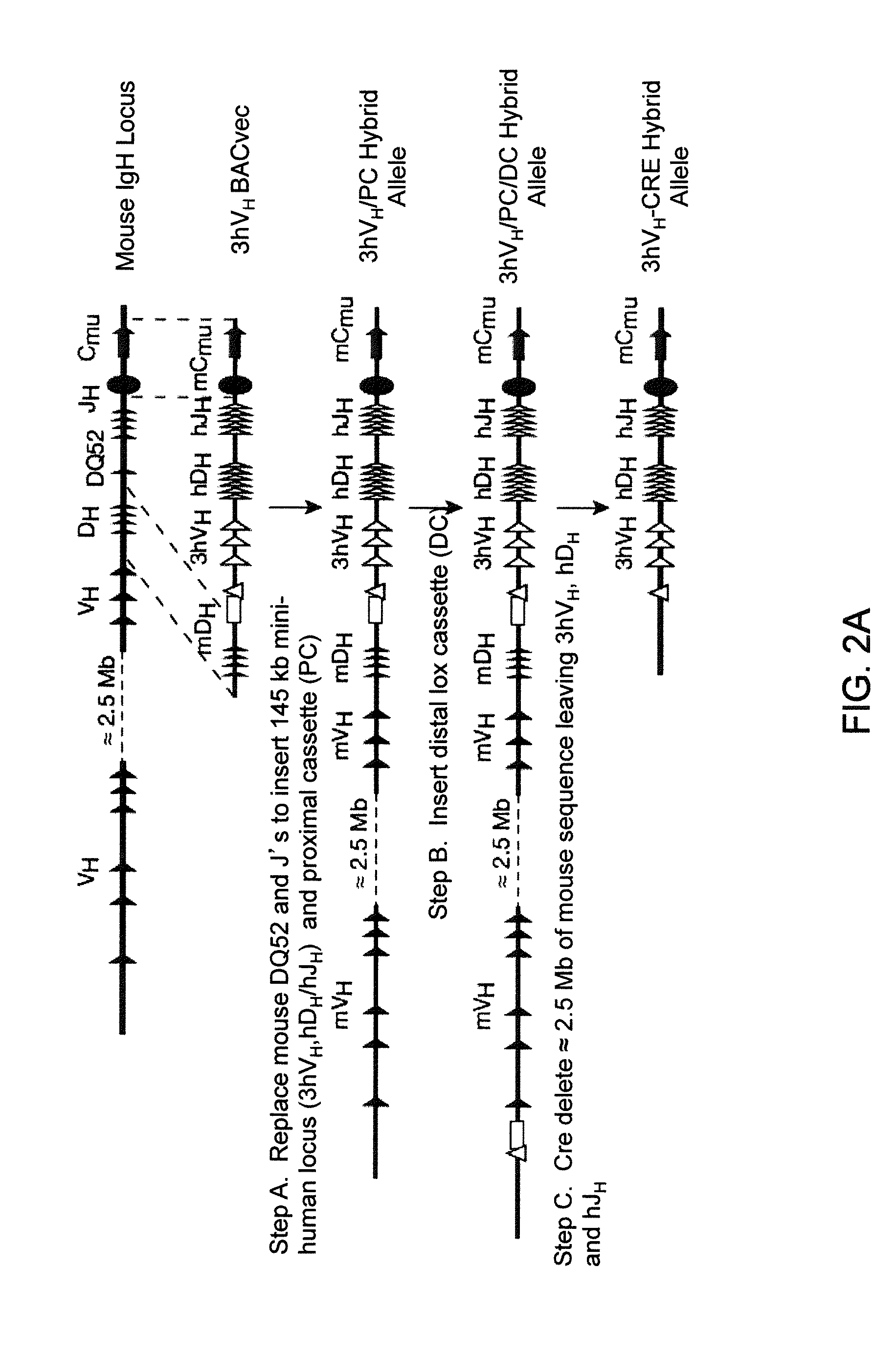
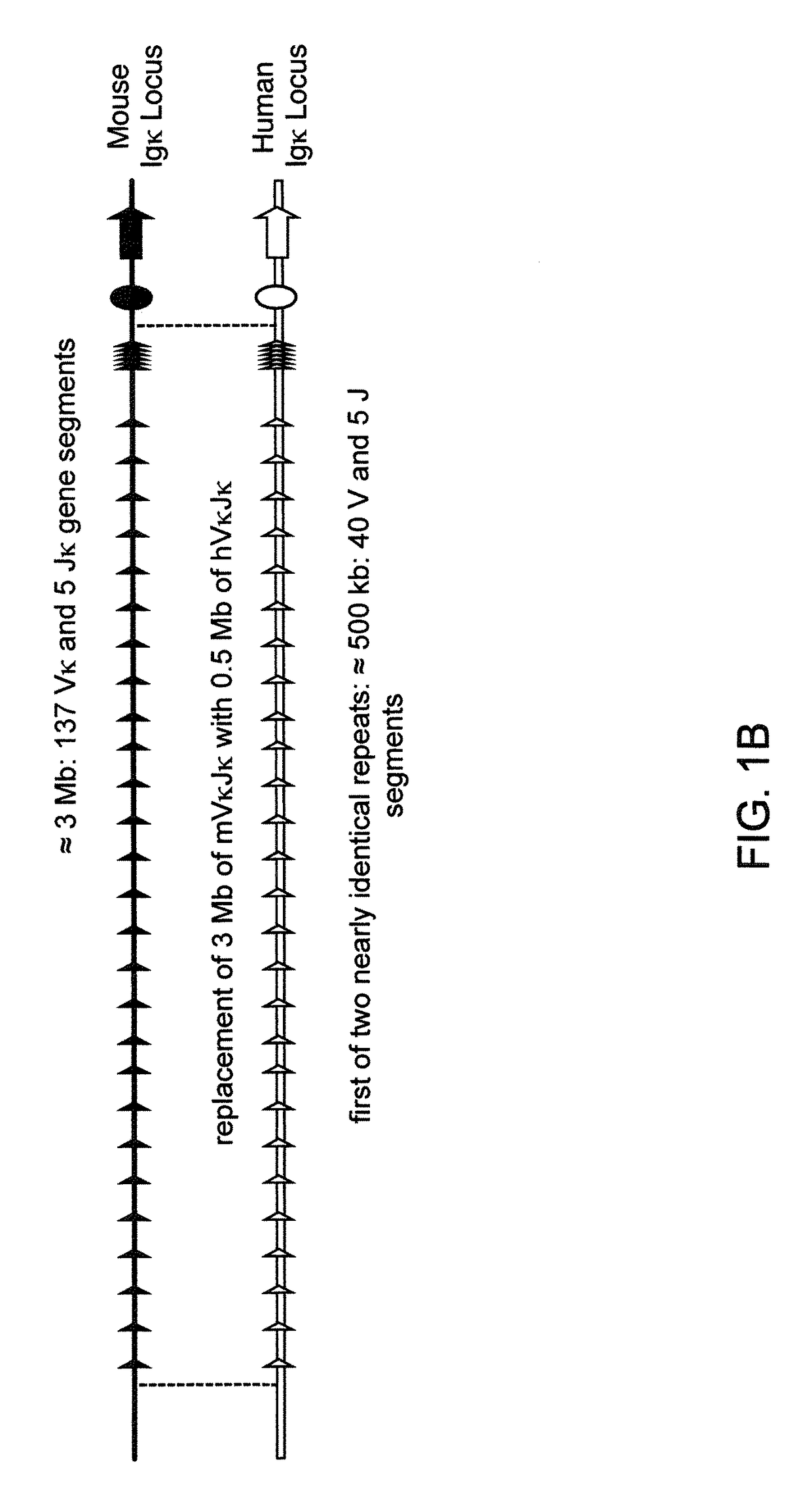
Transgenic mammals having human Ig loci including plural Vh and Vk regions and antibodies produced therefrom
InactiveUS20080098490A1Reduced development and maturation of B-cellsEfficient productionAntipyreticAnalgesicsHuman animalMammal
Owner:AMGEN FREMONT INC
Humanized Rodents that Express Heavy Chain Containing VL Domains
InactiveUS20130212719A1Reduced fertilityImprove fertilityAnimal cellsHybrid immunoglobulinsGenetic MaterialsVariable domain
Non-human animals, tissues, cells, and genetic material are provided that comprise a modification of an endogenous non-human heavy chain immunoglobulin sequence and that comprise an ADAM6 activity functional in a rodent (e.g., a mouse), wherein the non-human animals rearrange human immunoglobulin light chain gene segments in the context of heavy chain constant regions and express immunoglobulin-like molecules comprising human immunoglobulin light chain variable domains fused to heavy chain constant domains that are cognate with human immunoglobulin light chain variable domains fused to light chain constant domains.
Owner:REGENERON PHARM INC
Human TNF receptor fusion protein
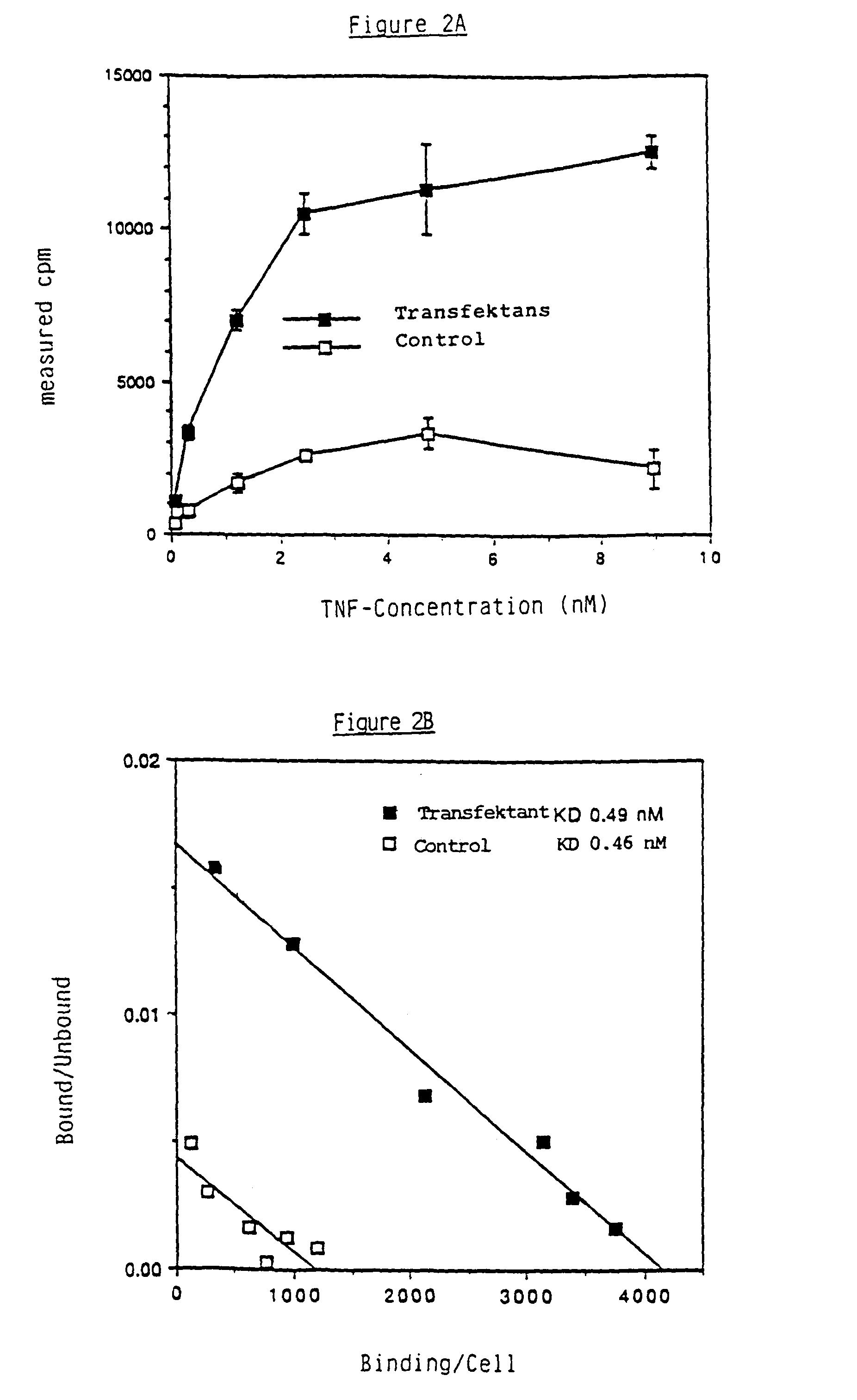
The present invention is concerned with non-soluble proteins and soluble or insoluble fragments thereof, which bind TNF, in homogeneous form, as well as their physiologically compatible salts, especially those proteins having a molecular weight of about 55 or 75 kD (non-reducing SDS-PAGE conditions), a process for the isolation of such proteins, antibodies against such proteins, DNA sequences which code for non-soluble proteins and soluble or non-soluble fragments thereof, which bind TNF, as well as those which code for proteins comprising partly of a soluble fragment, which binds TNF, and partly of all domains except the first of the constant region of the heavy chain of human immunoglobulins and the recombinant proteins coded thereby as well as a process for their manufacture using transformed pro- and eukaryotic host cells.
Owner:F HOFFMANN LA ROCHE INC
Transgenic animals and methods of use
The present invention comprises non-human vertebrate cells and non-human mammals having a genome comprising an introduced partially human immunoglobulin region, said introduced region comprising human VH coding sequences and non-coding VH sequences based on the endogenous genome of the non-human mammal.
Owner:TRIANNI INC
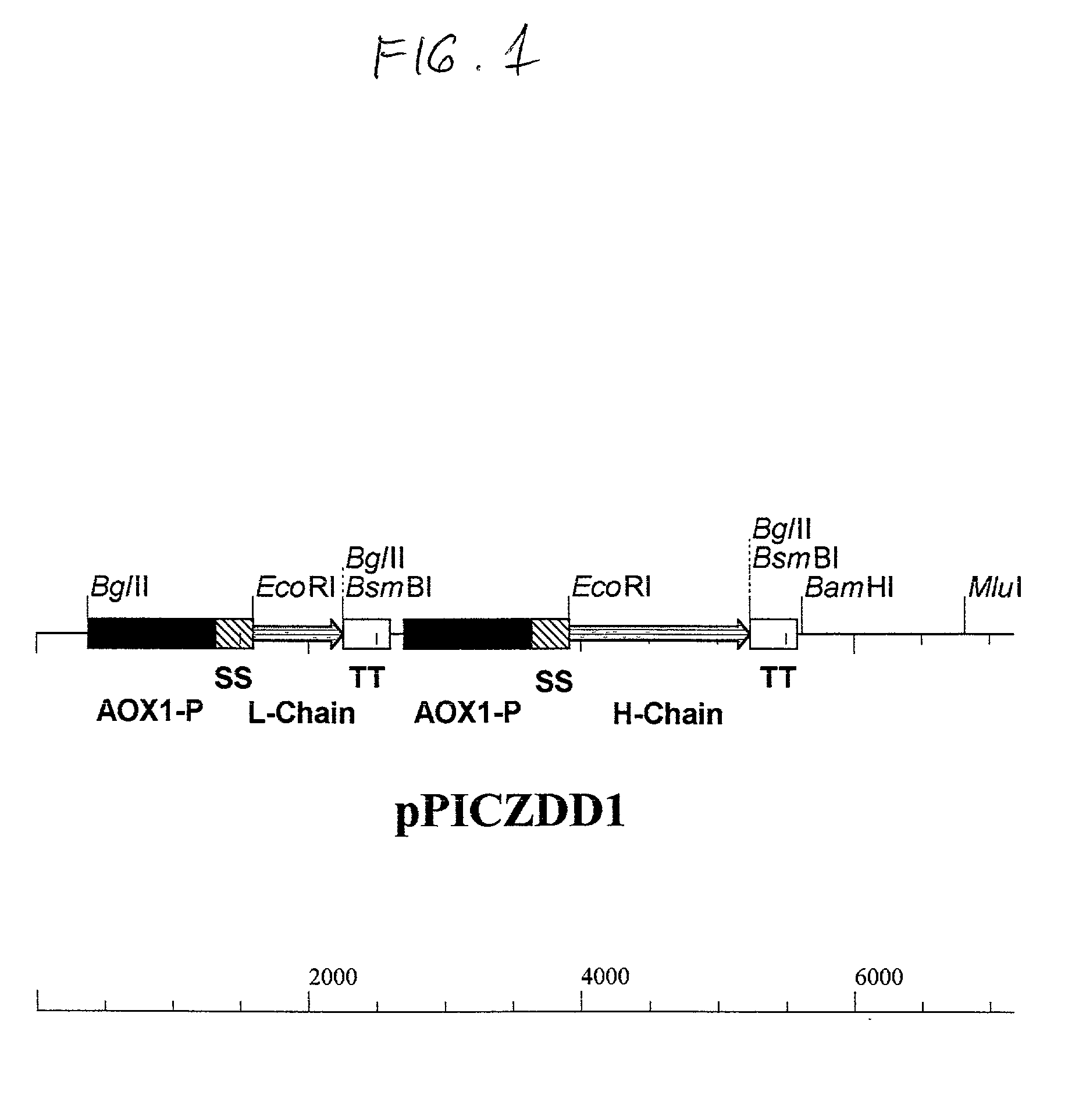
Functionally assembled antigen-specific intact recombinant antibody and a method for production thereof
Functionally assembled antigen-specific intact recombinant monoclonal antibody produced by transformation of the methylotropic yeast, P. pastoris with mouse / human immunoglobulin genes encoding heavy and light chains. A method for production of the intact monoclonal antibodies, a recombinant yeast expression vector and the antibody-specific MRNA synthesis. A process for a large-scale production of the functionally assembled intact recombinant antibody.
Owner:RGT UNIV OF CALIFORNIA
Humanized Light Chain Mice
ActiveUS20130160153A1Reduced fertilityImprove fertilityAnimal cellsNucleic acid vectorHuman immunoglobulinsGenetic Materials
Non-human animals, tissues, cells, and genetic material are provided that comprise a modification of an endogenous non-human heavy chain immunoglobulin sequence and that comprise an ADAM6 activity functional in a mouse, wherein the non-human animals express a human immunoglobulin heavy chain variable domain and a cognate human immunoglobulin λ light chain variable domain.
Owner:REGENERON PHARM INC
Transgenic animals for producing specific isotypes of human antibodies via non-cognate switch regions
InactiveUS20060134780A1Immunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenBeta globulins
The present invention provides fully human antibodies in a transgenic animal of a desired isotype in response to immunization with any virtually any desired antigen. The human immunoglobulin heavy chain transgene in the foregoing animals comprises a human constant region gene segment comprising exons encoding the desired heavy chain isotype, operably linked to switch segments from a constant region of a different heavy chain isotype, i.e., a non-cognate switch region. Said additional constant region segment comprises a switch region and human constant region coding segment, wherein the constant region coding segment is operably linked to a switch region that it is not normally associated with, i.e., a non-cognate switch region. In the transgenes of the invention, the non-cognate switch region may be a switch region from a different species than the constant region coding segment. The switch region and membrane exons of the invention may comprise a human gamma-2 constant region and the secreted constant region exons are from a human gamma-1 or a human gamma-4 constant region.
Owner:AMGEN FREMONT INC
Humanized light chain mice
ActiveUS20140017228A1Reduces and eliminates ADAM activityImprove fertilityNucleic acid vectorImmunoglobulinsHuman immunoglobulinsGenetic Materials
Non-human animals, tissues, cells, and genetic material are provided that comprise a modification of an endogenous non-human heavy chain immunoglobulin sequence and that comprise an ADAM6 activity functional in a mouse, wherein the non-human animals express a human immunoglobulin heavy chain variable domain and a cognate human immunoglobulin λ light chain variable domain.
Owner:REGENERON PHARM INC
Transgenic bovine comprising human immunoglobulin loci and producing human immunoglobulin
Owner:SAB LLC
Recombined human hyaluronidase, production and purification method and preparations thereof, use method and application
InactiveCN103468662AImprove permeabilityPharmaceutical non-active ingredientsEnzymesNasal cavityDisease
The invention discloses a recombined human hyaluronidase, a production and purification method and preparations of the recombined human hyaluronidase, a use method and application. Recombined human hyaluronidase PH20 or human hyaluronidase human albumin fusion protein PH20-HSA or human hyaluronidase human immunoglobulin IgG2Fc fragment fusion protein PH20-IgFc is adopted by the recombined human hyaluronidase and used in the mucosa or the surface of the skin. The preparations of the recombined human hyaluronidase can be made into different types such as membrane preparations, spray preparations, lotion and freeze-dried powder spray and used for skin infiltration promotion of beauty nutrient substances, skin mucosa infiltration promotion of surface anesthetic, infiltration promotion of skin disease therapeutic medicine, mucosa infiltration promotion of biological tranquillizer, mucosa skin infiltration promotion of growth factors, mucosa infiltration promotion of hypoglycemic drug, mucosa nasal cavity infiltration promotion of nervous centralis nutrient substances and the like.
Owner:惠觅宙
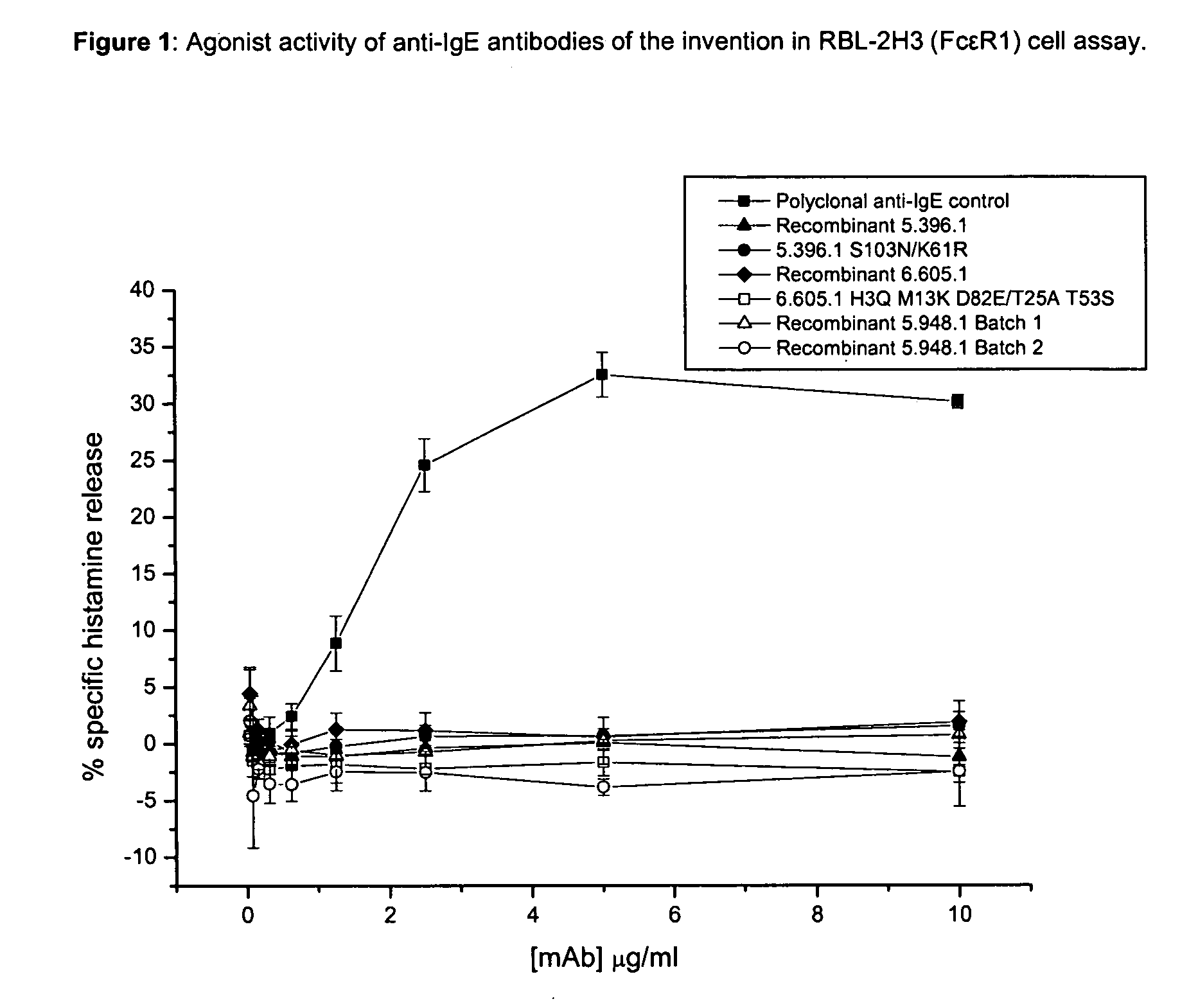
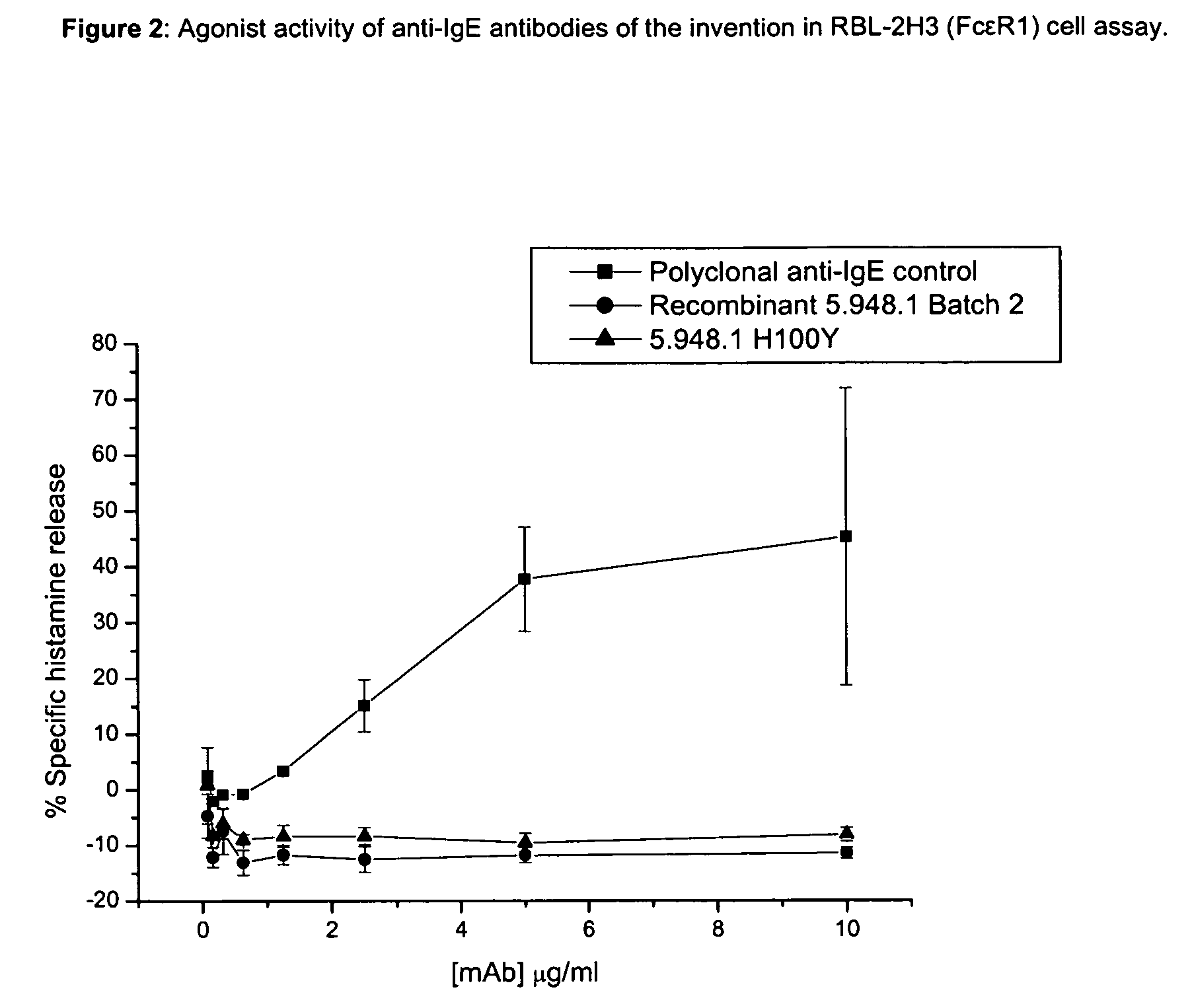
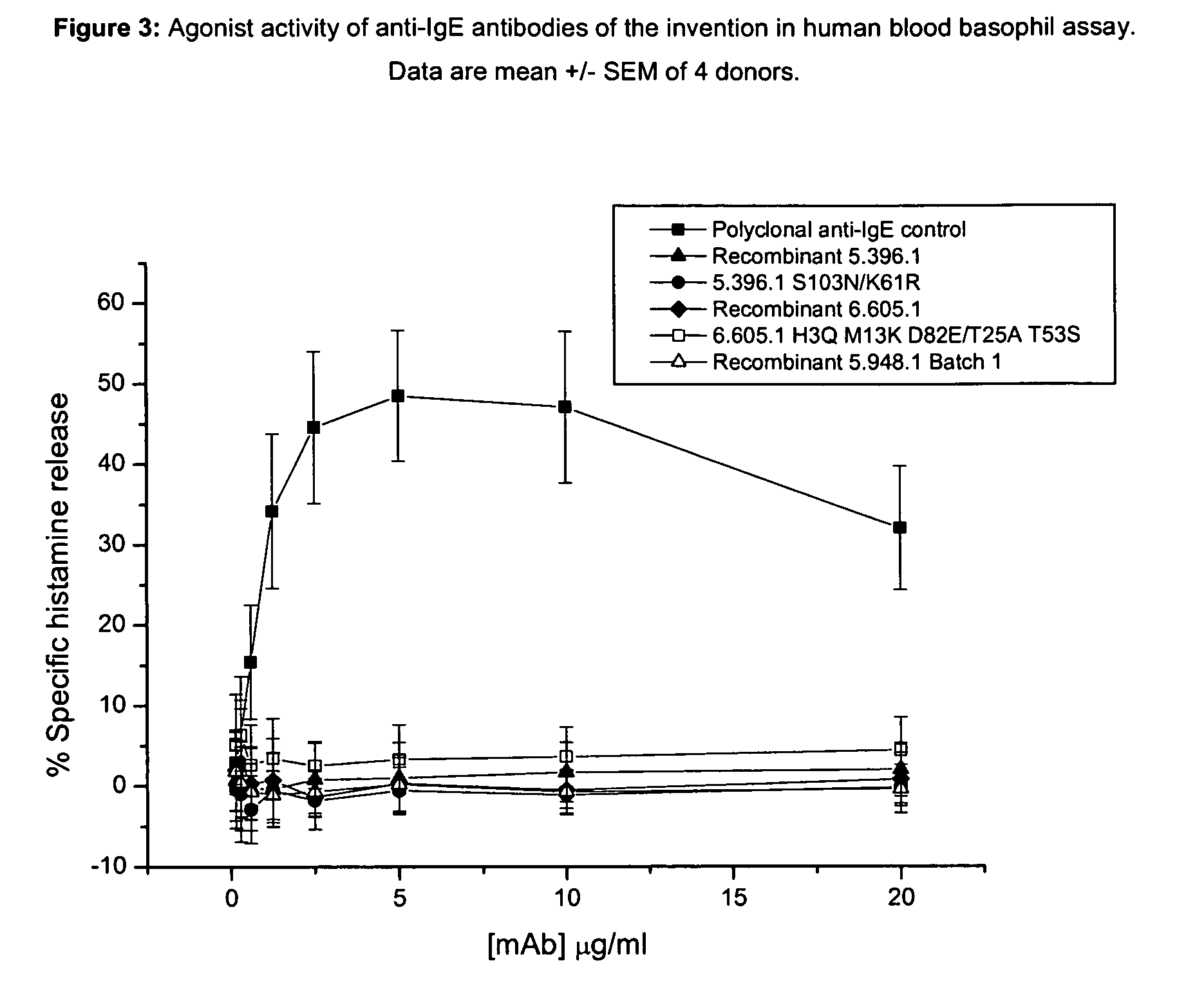
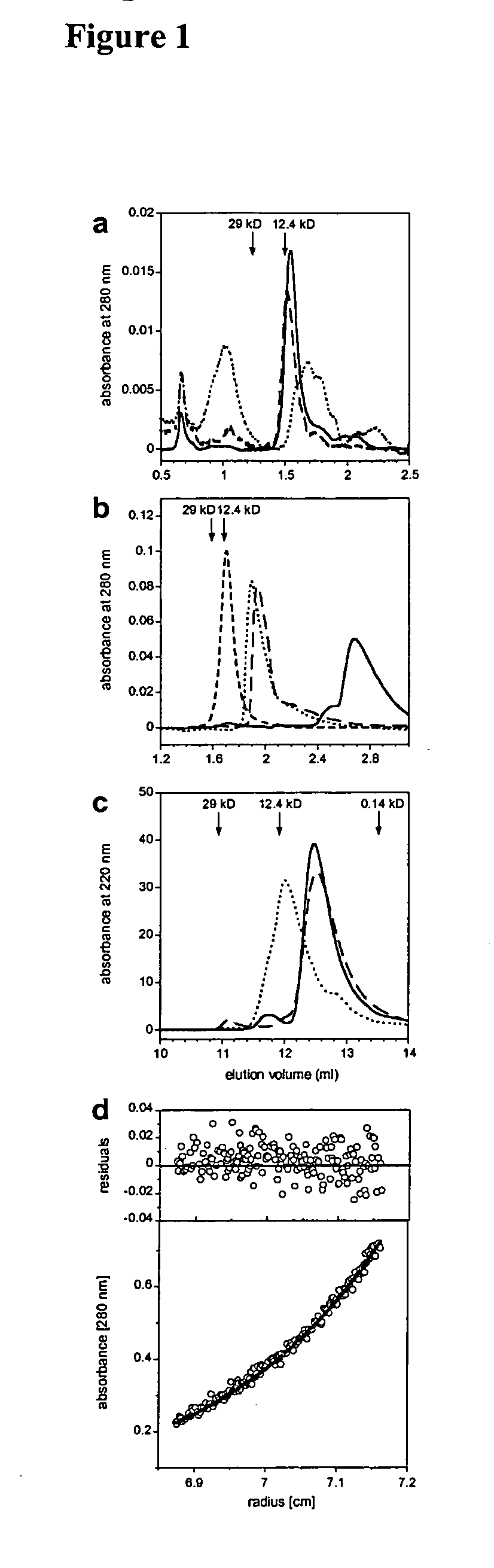
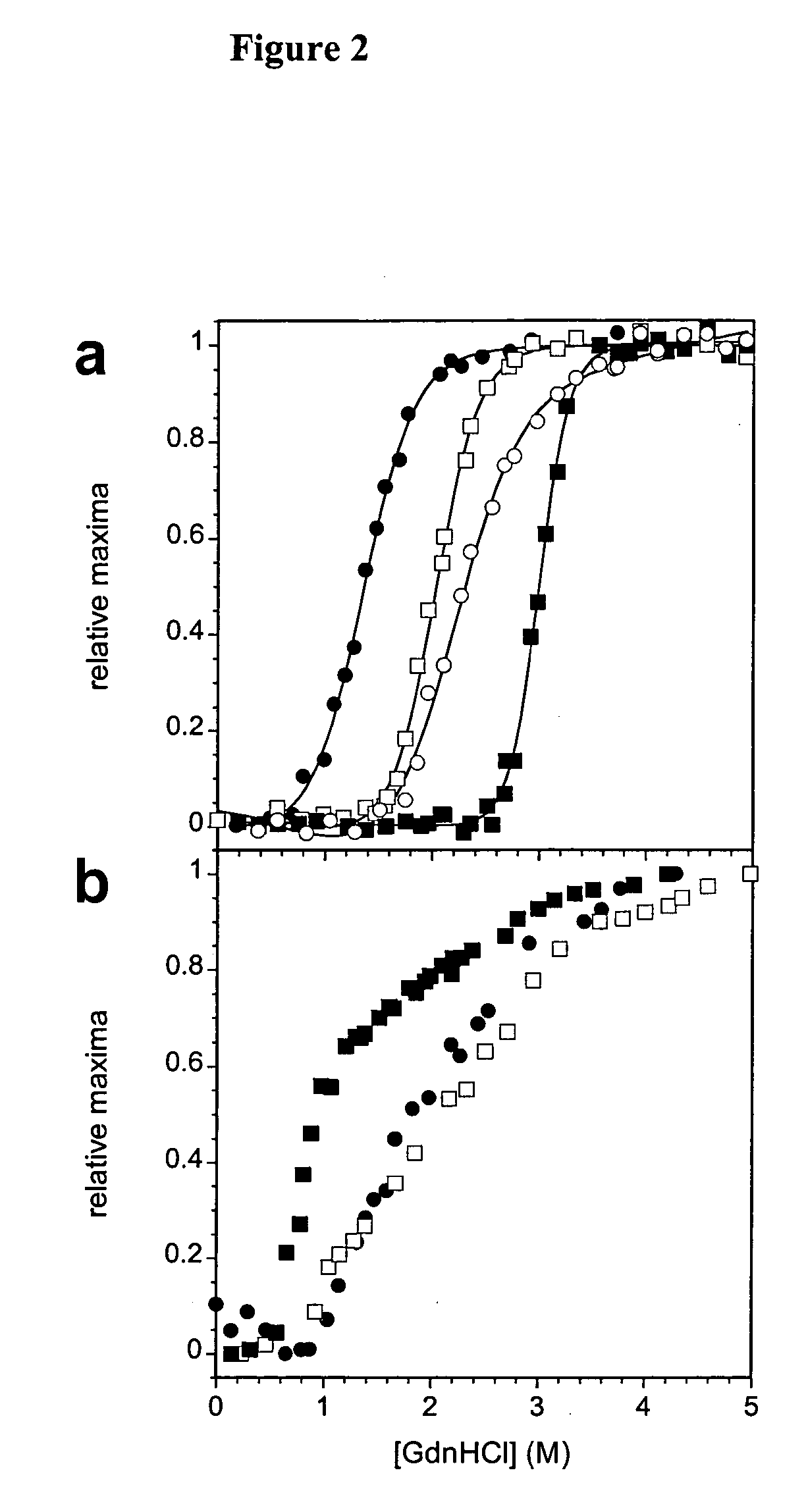
Anti-IgE antibodies
The present invention relates to novel human antibodies specifically directed against human immunoglobulin E (anti-IgE). The present invention also relates to pharmaceutical compositions and methods for treating asthma, in particular allergic asthma, as well as other IgE-mediated disorders including allergic rhinitis and food allergies.
Owner:PFIZER INC +1
Stabilized human immunoglobulin composition
InactiveUS20130216522A1Improve toleranceEasy to storeNervous disorderPeptide/protein ingredientsGlycineHuman immunoglobulins
The invention relates to a liquid pharmaceutical composition comprising human immunoglobulins G (IgGs), comprising at least 200 mM, preferably 250 mM±50 mM, of glycine and between 20 and 100 mg / l of a non-ionic detergent, and having a pH of less than or equal to 4.8.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Preparation process of intravenous injection human immunoglobulin
ActiveCN101972479AHigh yieldHigh purityAntibody ingredientsImmunoglobulinsUltrafiltrationPhosphoric acid
The invention relates to a preparation process of intravenous injection human immunoglobulin, belonging to the field of pharmaceuticals. On a basis of a traditional preparation process of intravenous injection human immunoglobulin with the low protein concentration of 5 percent, a filter pressing method is adopted instead of a centrifuging method in an extraction process. During hyperfiltration, the protein concentration is adjusted to 3-6 percent, a pH value is adjusted to 6.4-6.6 with 0.5 mol / L of NaOH; then, 1 mol / L phosphoric acid-NaOH buffer solution is added to adjust the electrical conductivity which is measured to be 0.175-0.205 s / m at a temperature T of 19 DEG C; and a chromatography method is adopted to carrying out column chromatography and purification by using upper ion exchange columns after the electrical conductivity is adjusted. The protein impurities can be effectively removed, the protein purify and the product yield are improved; maltose or glycin is used as a protector, which benefits the improvement of the stability of the intravenous injection human immunoglobulin; and the glycin is used as the protector, which satisfies the clinical use of diabetics. According to the invention, the intravenous injection human immunoglobulin with the protein concentration of 5-11 percent can be obtained.
Owner:华润博雅生物制药集团股份有限公司
Human TNF receptor
Owner:F HOFFMANN LA ROCHE & CO AG
Human heavy chain antibody expression in flamentous fungi
InactiveUS20060234340A1Efficient expressionReduce solubilityFungiSugar derivativesProtein CHeavy-chain antibody
The present invention relates to a method for producing a functional human immunoglobulin, wherein a human heavy chain immunoglobulin, devoid of any light chain, is expressed, comprising the steps of: a) transforming a filamentous host cell with a recombinant construct encoding a modified human heavy chain immunoglobulin, wherein the modifications comprise one or more mutations in the region of the heavy chain protein involved in contact with the light chain; b) culturing said filamentous host cell under conditions promoting expression of said modified human heavy chain immunoglobulin; and c) recovering said modified human heavy chain immunoglobulin.
Owner:NOVOZYMES AS
Modification of human variable domains
InactiveUS20060127893A1Increasing TmImprove stabilityPeptide librariesFungiHuman immunoglobulinsVariable domain
The present invention relates to a method for the optimization of isolated human immunoglobulin variable heavy (VH) and light (VL) constructs.
Owner:UNIV ZURICH
Concentrated human immunoglobulin composition
InactiveUS20130121991A1Easy to administer subcutaneouslyGood flexibilityAntibacterial agentsNervous disorderHuman immunoglobulinsHuman Immunoglobulin G
The invention relates to a human immunoglobulin G composition characterized in that the human immunoglobulin G concentration is at least 230 g / l, which is of use in particular for subcutaneous administration.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Recombinant varicella zoster virus vaccine
ActiveCN112870344AHigh molecular weightImproving immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsChickenpoxImmunogenicity
The invention discloses a recombinant varicella zoster virus vaccine, which comprises an amino acid sequence of a recombinant glycoprotein gE extracellular region of a live attenuated VZV strain (OKA strain) gene and a fusion protein formed by a human immunoglobulin Fc segment, and further comprises preparation and an application of the fusion protein, and a corresponding recombinant gene, an eukaryotic expression vector and the like. The fusion protein provided by the invention has good immunogenicity, and can induce generation of a high-level serum neutralizing antibody.
Owner:BEIJING LUZHU BIOTECH +1
Hyperglycosylated human growth hormone fusion protein and preparation method and application thereof
InactiveCN106256835AImprove stabilityLow immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeImmunoglobulin Fc Fragments
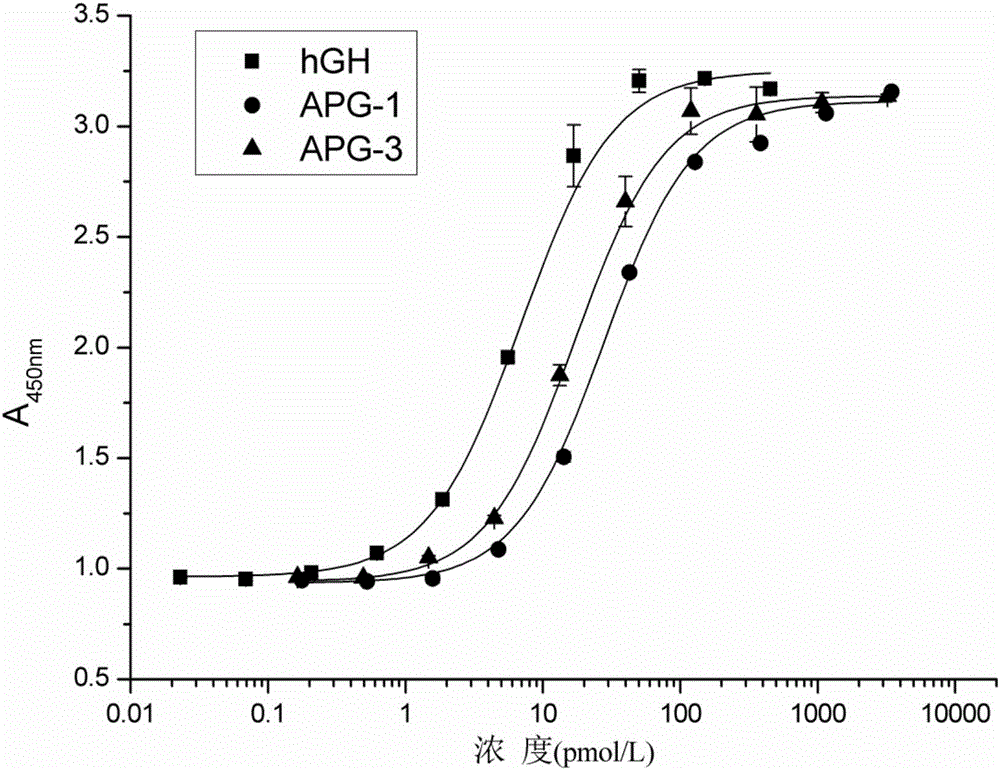
The invention discloses hyperglycosylated human growth hormone fusion protein. The human growth hormone fusion protein sequentially contains a human growth hormone (hGH), a flexible peptide joint (L), human chorionic gonadotropin beta-carboxyl terminal rigid peptide (CTP) and a human immunoglobulin Fc fragment from the N terminal to the C terminal. The invention further discloses a method for efficiently preparing the fusion protein. Compared with a recombinant hGH, the built fusion protein has more excellent in-vivo drug efficacy, the in-vivo circulation half-life period is prolonged, the administration frequency is greatly decreased, and the bioavailability is improved; meanwhile, the production process is simpler and more efficient.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
Humanized light chain mice
ActiveUS9622459B2Reduces and eliminates ADAM activityImprove fertilityHybrid immunoglobulinsHydrolasesHuman immunoglobulinsGenetic Materials
Owner:REGENERON PHARM INC
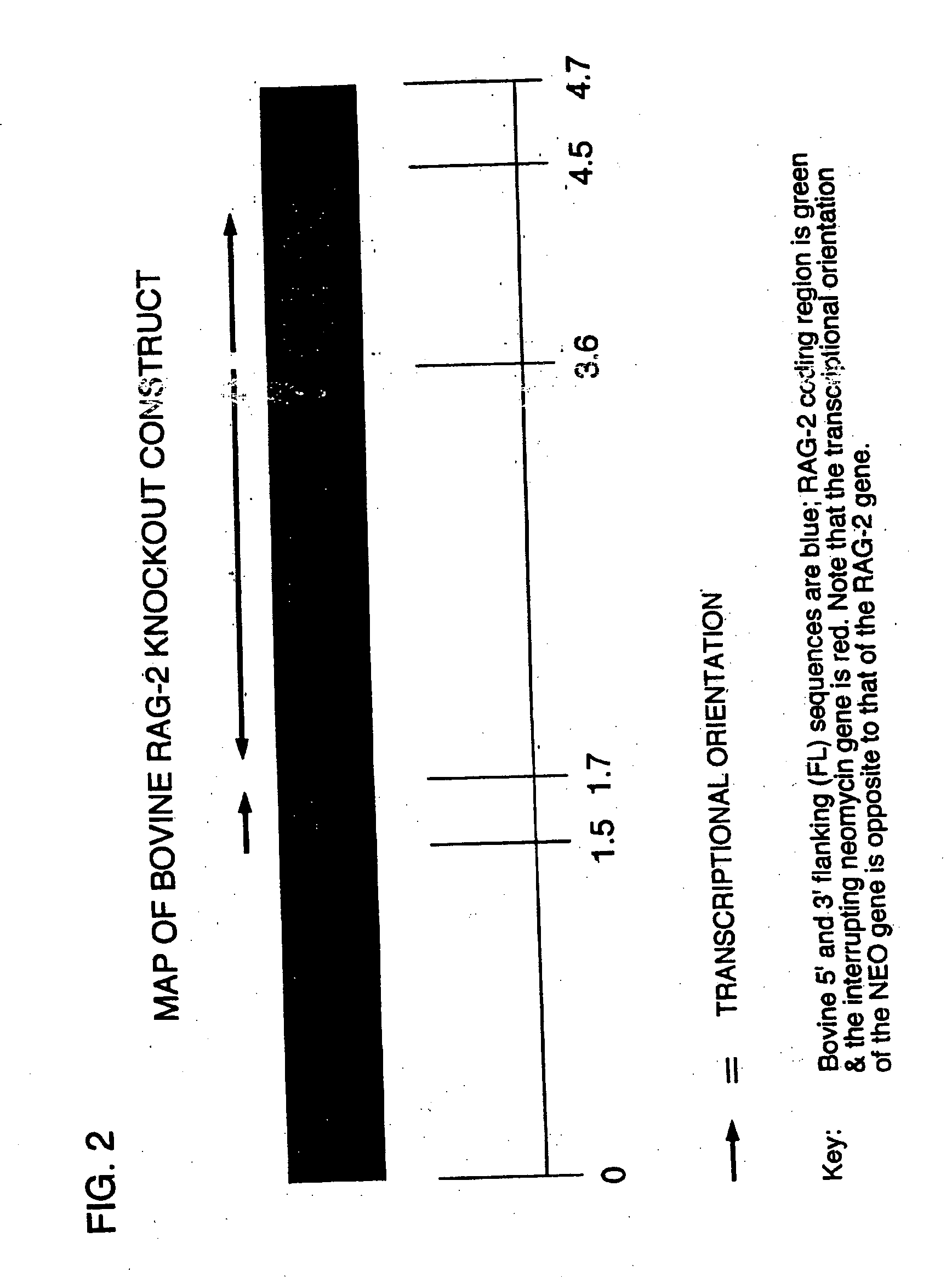
Production of ungulates, preferably bovines that produce human immunoglobulins
The present invention relates to a method of producing an ungulate having both copies of the IgM heavy chain (mu) rag-1 and / or rag-2 gene eliminated from its genome. Animals which have IgM, rag-1 and / or rag-2 eliminated from their genome are unable to conduct the gene rearrangements that are necessary to generate the antigen receptors of B or T lymphocytes, and therefore will not develop native B or T cells. Because they are unable to produce B and T lymphocytes, these IgM, rag-1 or rag-2 ungulates cannot reject human hematopoietic stem cell preparations, and B and T lymphocytes which develop therefrom. Therefore, the present invention also involves injecting into IgM, rag-1 and / or rag-2 deficient ungulates, in utero or shortly after birth, human B and T lymphocytes whose immune systems produce human immunoglobulin that can be processed for therapeutic uses in humans.
Owner:KYOWA HAKKO KIRIN CO LTD
COVID-19 subunit vaccine as well as preparation method and application thereof
ActiveCN113321739AEasy extractionEasy to purifySsRNA viruses positive-senseViral antigen ingredientsAdjuvantReceptor
Fusion protein RBD-hFc / RBD-His obtained by combining a novel coronavirus (SARS-CoV-2) spike protein receptor binding structural domain (RBD) with a human immunoglobulin hFc domain or His tag through a genetic engineering means is convenient to extract and purify, stable and controllable in quality, short in time consumption and capable of being produced on a large scale. A vaccine composition prepared from the fusion protein and an adjuvant can increase the solubility and stability of a vaccine, enhance the immunogenicity of the vaccine and prolong the half-life period of the vaccine in vivo. The fusion protein and the vaccine composition thereof can inhibit replication and transmission of SARS-CoV-2 wild type and / or variant strains or prevent the SARS-CoV-2 wild type and / or variant strains from settling in a host, so that novel coronavirus pneumonia caused by the SARS-CoV-2 wild type and / or variant strains can be effectively prevented and / or treated.
Owner:广东克冠达医药科技有限公司
LIQUID FORMULATIONS FOR TNFR:Fc FUSION PROTEINS
ActiveUS20150290325A1Peptide/protein ingredientsAntibody mimetics/scaffoldsHuman immunoglobulinsADAMTS Proteins
The invention provides stable liquid formulations for a recombinant biopharmaceutical protein comprising a soluble form of the human p75 TNF receptor fused to an Fe domain of a human immunoglobulin protein (TNFR:Fc). Typically, biopharmaceutical proteins such as monoclonal antibodies (mAbs) and immunoglobulin fusion proteins (e.g., immunoadhesion proteins) are produced by recombinant DNA technology in mammalian cell expression systems. In order to guarantee the reproducible clinical performance of a biopharmaceutical product, manufacturers have to deliver a product of consistent and reproducible quality.
Owner:MERCK SHARP & DOHME CORP
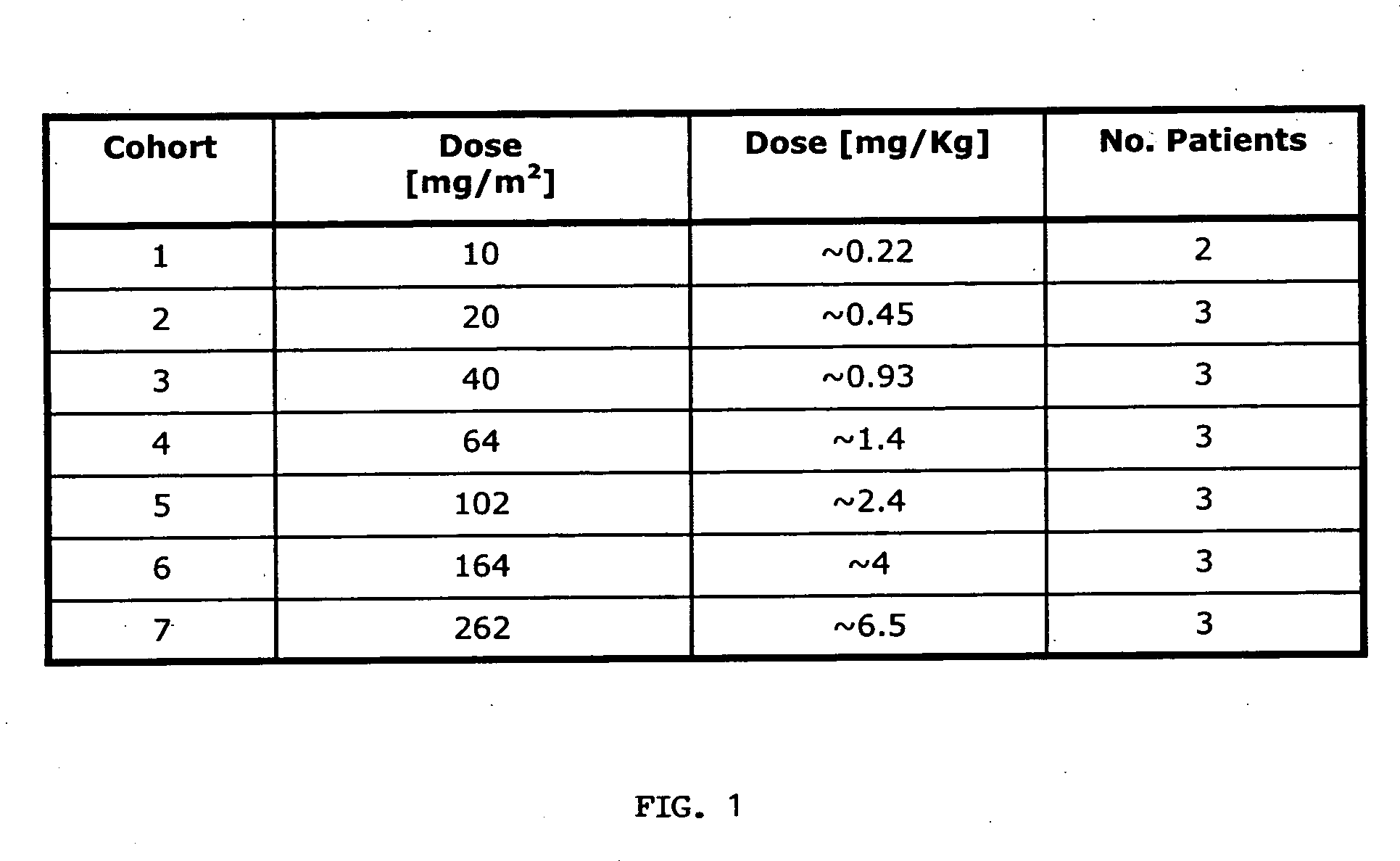
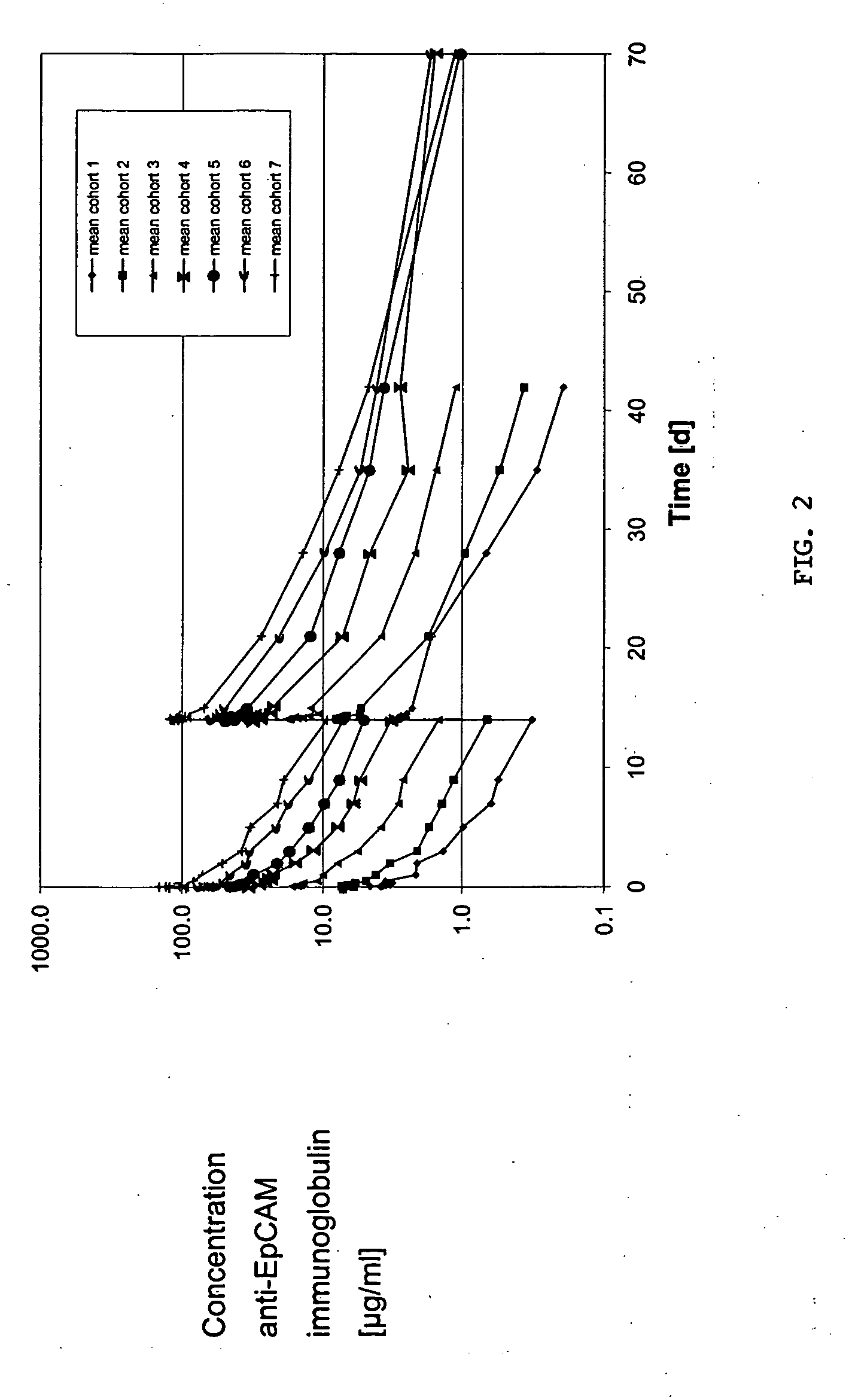
Anti-EpCAM immunoglobulins
InactiveUS20050180979A1Improve durabilityEliminating of least mitigating adverseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHuman immunoglobulinsHalf-life
The invention relates inter alia to a method of treating tumorous disease in a human patient by administering to the patient a human immunoglobulin specifically binding to the human EpCAM antigen, the immunoglobulin exhibiting a serum half-life of at least 15 days, the method comprising the step of administering the immunoglobulin no more frequently than once every week, preferably no more frequently than once every two weeks.
Owner:MICROMET AG
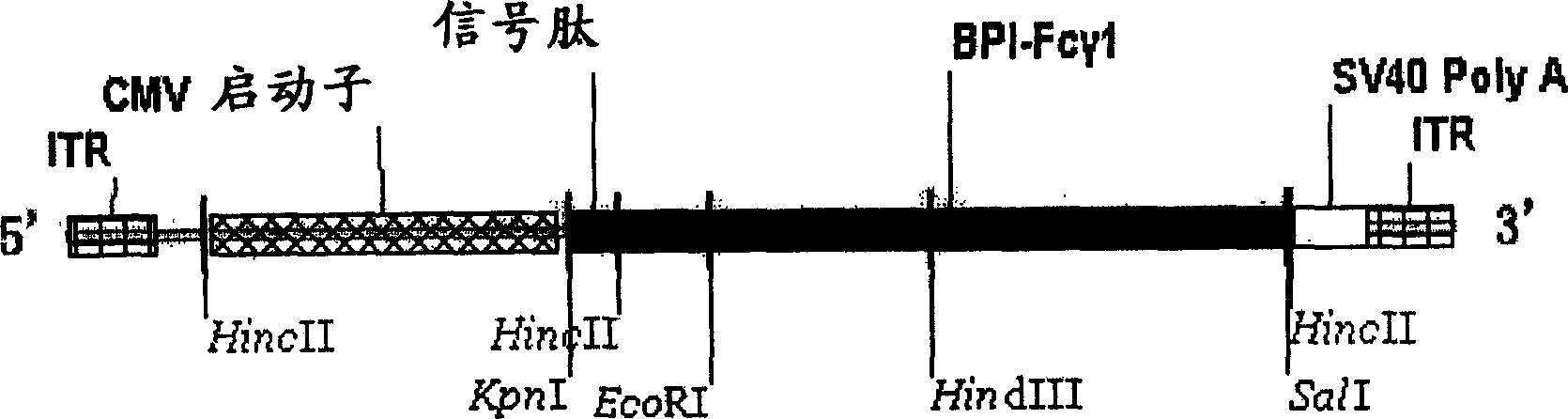
Recombinant virus containing BPI gene and pharmaceutical composition containing same and uses
The invention relates to a recombinant virus, which comprises viral vector and genes construct selected from: (1) human BPI gene or its functional fragment gene, or its degenerate sequence, (2) chimeric gene containing human BPI gene or its functional fragment gene, or its degenerate sequence, wherein the 3' end of the human BPI gene or its functional fragment gene is further connected to human immunoglobulin heavy chain constant region Fc gene or its equivalent gene. The invention also relates to the use of the gene construct containing human BPI gene or its functional fragment gene for preparing pharmaceutical composition for the gene therapy of mammal GNB and / or GNB-like pathogen infectious diseases. The invention also relates to the method for treating GNB and / or GNB-like pathogen infectious diseases by utilizing the recombinant virus or pharmaceutical composition.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES +1
Human urine immunoglobulin G detection kit based on latex-enhanced immunoturbidimetry
InactiveCN109633172AImprove stabilityGood repeatabilityBiological testingHuman immunoglobulinsMicrosphere
The invention, which belongs to the technical field of medical in-vitro diagnostics, relates to a human urine immunoglobulin G detection kit based on latex-enhanced immunoturbidimetry, thereby solvinga problem of providing a human urine immunoglobulin G content detection kit having advantages of wide detection linear range, high detection accuracy, and high detection stability. The kit comprisesan R1 reagent, an R2 reagent and a calibrator. The R1 reagent includes a buffer solution, NaCl and a preservative. The R2 reagent includes a buffer solution, an antibody-coupling latex microsphere, aprotective agent and a preservative; and the antibody is the goat anti-human immunoglobulin polyclonal antibody or a rabbit anti-human immunoglobulin polyclonal antibody. The calibrator is formed by aplurality of solution; each solution includes human immunoglobulin G, a buffer solution and a preservative; and the concentrations of the human immunoglobulin G in different solutions are different.The kit has advantages of wide detection linear range, high detection accuracy, and high detection stability.
Owner:DIRUI MEDICAL TECH CO LTD
Surface antigen 1 of Toxoplasma gondii human antibody Fab fragment and encoded gene thereof
The present invention belongs to the field of biotechnology, and relates to a surface antigen 1 (SAG1) of Toxoplasma gondii human antibody Fab fragment, encoded gene and use thereof. According to the invention, the surface antigen 1 (SAG1) of Toxoplasma gondii human antibody Fab fragment is filtered from a base through establishing a Toxoplasma gondii human immunoglobulin, ELISA, diluting the prothrombin time, sequencing analysis, etc. Through expression purifying and authenticating, the human antigen Fab fragment is authenticated to specifically identify the tachyzoite-bradyzoite recombination SAG1 of Toxoplasma gondii and have higher affinity with the tachyzoite-bradyzoite recombination SAG1 of Toxoplasma gondii, for being identified with the specificity of Toxoplasma gondii tachyzoite-bradyzoite. The human antigen Fab fragment of the invention does not contain Fc segment and does not activate the alexin or cause the histopathological damages of human immune response, etc. when the function of restricting the invasion of Toxoplasma gondii to the host cell is exerted. The surface antigen 1 (SAG1) of Toxoplasma gondii human antibody Fab fragment is safe and reliable when applied for the human body. The antigen medicine for treating toxoplasmosis or the antigen targeted medicine can be prepared.
Owner:FUDAN UNIV
Catalytic Immunoglobulins BBK32 and Uses Therefor
InactiveUS20090297534A1Delay progressReduce resistanceAntibacterial agentsNervous disorderHuman immunoglobulinsStereochemistry
The present invention describes the composition of class and subclass selected pooled human immunoglobulins with catalytic activity, methods of preparation thereof, and therapeutic utility thereof.
Owner:PAUL SUDHIR +3
New application of rabies human immunoglobulin in preparing medicine for preventing and controlling Japanese encephalitis and combined vaccine for rabies and Japanese encephalitis
InactiveCN101524539AReduce the number of vaccinationsVaccination promotion is easyViral antigen ingredientsAntiviralsJapanese encephalitis vaccineHuman immunoglobulins
The invention belongs to the field of biological products, in particular to a new application of the existing product-rabies human immunoglobulin. The technical proposal of the invention is the new application of rabies human immunoglobulin in preparing medicine for preventing and controlling Japanese encephalitis. The invention also provides a combined vaccine for rabies and Japanese encephalitis. The vaccine of the invention has better effect than conventional Japanese encephalitis vaccine in preventing and controlling Japanese encephalitis and fundamental immunity for resisting the rabies virus is obtained. The combined vaccine features scientific immunization schedule, few times of vaccine inoculation, easy promotion of inoculation and good effect in boostering immunization in case of emergency in preventing and controlling the rabies. The antibody and the vaccine jointly solve the technical and application problems of preventing and controlling the Japanese encephalitis and the rabies, thus enjoying great social and economic significance.
Owner:魏宪义
Humanized immunoglobulins
InactiveUS20080160018A1Maximize likelihoodHigh binding affinityFungiBacteriaImmunoglobulin heavy chainHuman immunoglobulins
Novel methods for producing, and compositions of, humanized immunoglobulins having one or more complementarity determining regions (CDR's) and possible additional amino acids from a donor immunoglobulin and a framework region from an accepting human immunoglobulin are provided. Each humanized immunoglobulin chain will usually comprise, in addition to the CDR's, amino acids from the donor immunoglobulin framework that are, e.g., capable of interacting with the CDR's to effect binding affinity, such as one or more amino acids which are immediately adjacent to a CDR in the donor immunoglobulin or those within about 3 Å as predicted by molecular modeling. The heavy and light chains may each be designed by using any one or all of various position criteria. When combined into an intact antibody, the humanized immunoglobulins of the present invention will be substantially non-immunogenic in humans and retain substantially the same affinity as the donor immunoglobulin to the antigen, such as a protein or other compound containing an epitope.
Owner:PDL BIOPHARMA INCORPORATED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com