Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Human Immunoglobulin G" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunoglobulin G (IgG) is a monomeric immunoglobulin, built of two heavy chains and two light chains. Each molecule has two antigen binding sites. It is the most abundant immunoglobulin and is approximately equally distributed in blood and in tissue liquids, and is the class of immunoglobulin characterized by heavy chains.
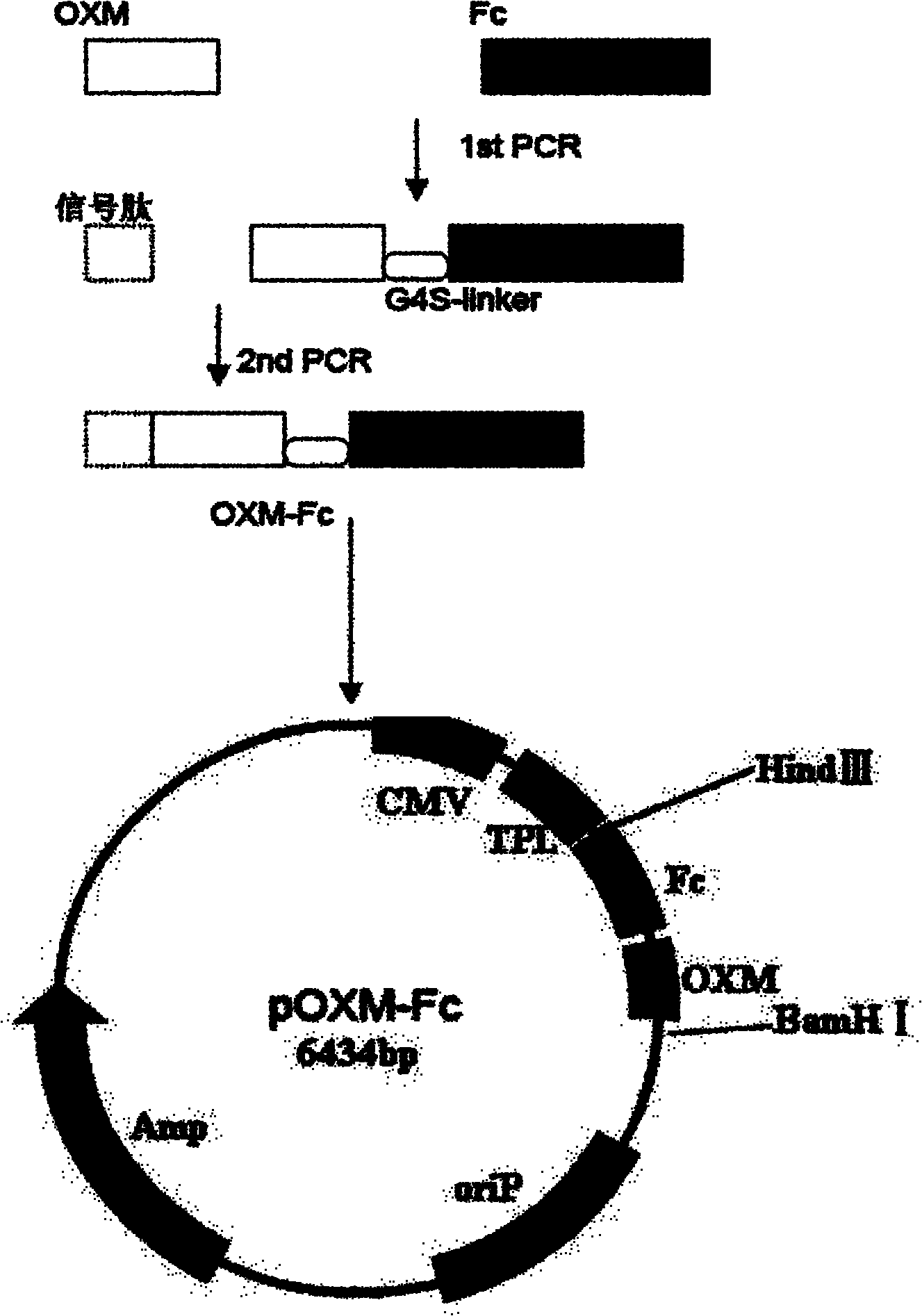
Recombinant oxyntomodulin (OXM) fusion protein, and preparation and application thereof
InactiveCN102010473AExtended half-lifeImprove stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseRecombinant expression
The invention relates to long-acting and stable oxyntomodulin (OXM). An Fc segment of human immunoglobulin G and the human OXM form fusion protein through a connecting peptide. A method for preparing the fusion protein comprises the following steps of: preparing the human OXM and an Fc segment gene of the human immunoglobulin respectively, and connecting the human OXM and the Fc segment gene of the human immunoglobulin to construct connecting segment-containing recombinant expression vectors; and transforming host cells by using the recombinant expression vectors, culturing the host cells and recovering from cell culture and purifying the host cells to obtain the recombinant fusion protein. The fusion protein can be used for preparing medicaments for treating metabolic diseases such as diabetes and obesity.
Owner:曹鹏 +1
Stabilized human immunoglobulin composition
InactiveUS20130216522A1Improve toleranceEasy to storeNervous disorderPeptide/protein ingredientsGlycineHuman immunoglobulins
The invention relates to a liquid pharmaceutical composition comprising human immunoglobulins G (IgGs), comprising at least 200 mM, preferably 250 mM±50 mM, of glycine and between 20 and 100 mg / l of a non-ionic detergent, and having a pH of less than or equal to 4.8.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
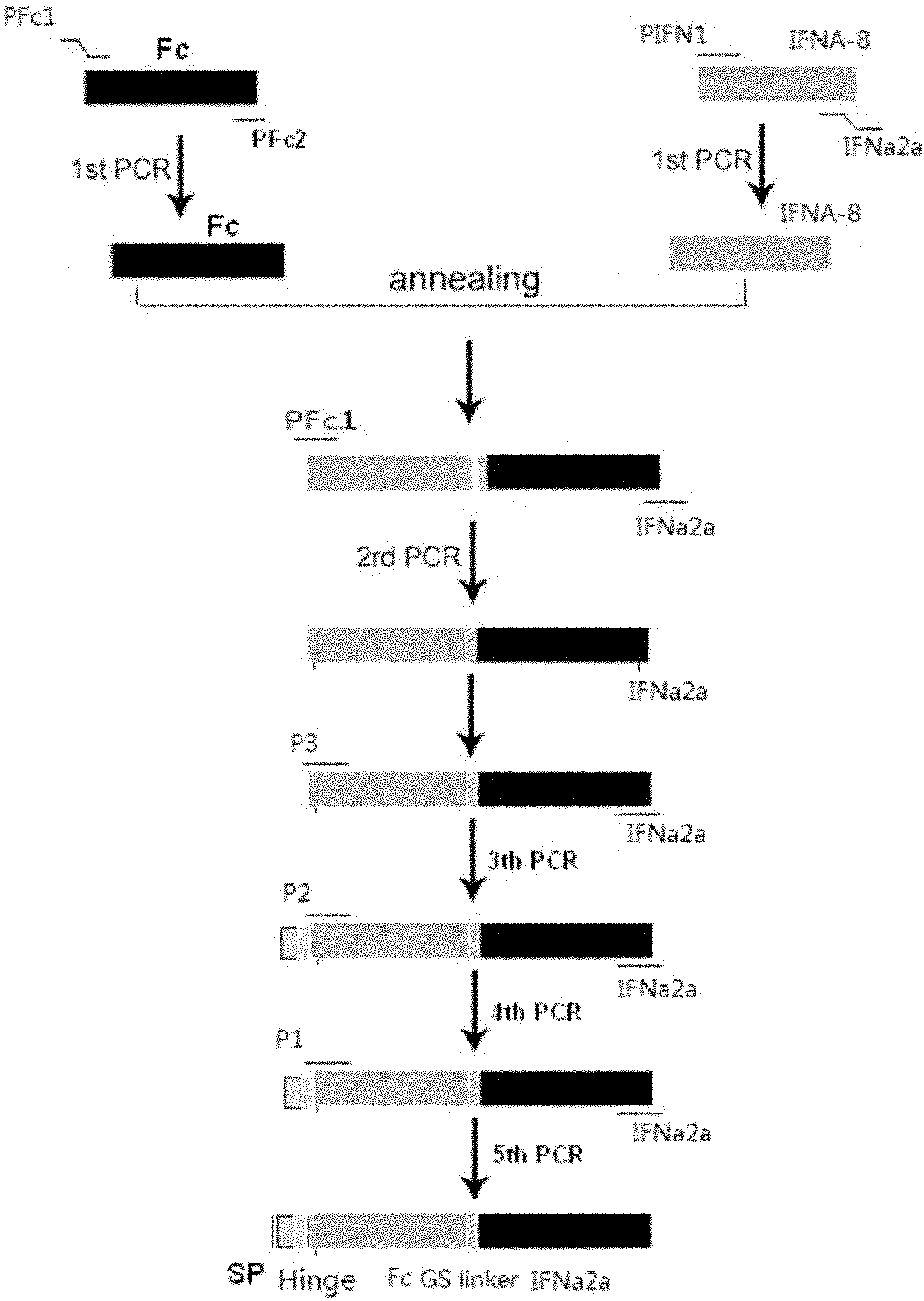
Interferon fusion protein, preparation thereof and application thereof
InactiveCN101967196AExtended half-lifeImprove stabilityPeptide/protein ingredientsAntiviralsIntravenous gammaglobulinFc fragment
The invention relates to an interferon fusion protein, preparation thereof and application thereof. The interferon fusion protein is a fusion protein formed by connecting an Fc fragment of the human immunoglobulin G and the human interferon, or connecting the human interferon and the Fc fragment of the human immunoglobulin through a connecting peptide. The preparation of the fusion protein comprises the following steps of: preparing an interferon gene and an Fc fragment gene of the human immunoglobulin G respectively, connecting the interferon gene and the Fc fragment gene and then constructing a recombinant expression vector which contains the connecting fragment; and transforming host cells by using the recombinant expression vector, culturing the host cells, recovering the recombinant fusion protein from cell culturing substances and purifying the recovered product to obtain the recombinant fusion protein. The fusion protein in the invention can be applied in the preparation of antivirus medicaments, antitumor medicaments and immune adjusting medicaments.
Owner:夏志南 +1
Treatment of alzheimer's disease subpopulations with pooled immunoglobulin g
The present invention provides, among other aspects, methods for the treatment of Alzheimer's disease in a subject in need thereof, the method including administration of a therapeutically effective amount of a pooled human immunoglobulin G (IgG) composition to a subject with moderately severe Alzheimer's disease, a subject carrying an ApoE4 allele, or both, where the amount of pooled human IgG is from 300 mg / kg to 800 mg / kg body weight of the subject per two week period, and where the amount is administered in one or more doses during the two week period after initiation of a therapeutic regimen. Also provided, are methods for selecting a treatment regimen for a subject with Alzheimer's disease, including diagnosing the severity of the Alzheimer's disease, determining if the subject carries an APOE4 allele, or both, and assigning a treatment regimen including administration of pooled human immunoglobulin G and / or an anti-beta amyloid monoclonal antibody.
Owner:BAXALTA INC
Concentrated human immunoglobulin composition
InactiveUS20130121991A1Easy to administer subcutaneouslyGood flexibilityAntibacterial agentsNervous disorderHuman immunoglobulinsHuman Immunoglobulin G
The invention relates to a human immunoglobulin G composition characterized in that the human immunoglobulin G concentration is at least 230 g / l, which is of use in particular for subcutaneous administration.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
STABLE LIQUID FORMULATION OF FUSION PROTEIN WITH IgG Fc DOMAIN
ActiveUS20160376342A1Long-term stabilityReduce storage conditionsSenses disorderPeptide/protein ingredientsDiseaseVEGF receptors
A stable liquid formulation includes a fusion protein having an Fc domain of a human immunoglobulin G (IgG), in particular, a protein in which an Fc domain of a human immunoglobulin G (IgG) and a soluble extracellular domain of a vascular endothelial growth factor (VEGF) receptor are fused (e.g., aflibercept)). A composition for stabilizing a protein and a method for stabilizing a protein in which an Fc domain of an IgG and a soluble extracellular domain of a VEGF receptor are fused are disclosed. The present invention improves therapeutic effects on various ophthalmic diseases (e.g., retinal vein occlusion, diabetic macular edema, choroidal neovascularization and wet age-related macular degeneration, etc.) caused by abnormal angiogenesis, while pursuing stabilization of bioactivity through a stable liquid formulation suitable for intravitreal injection of an anti-VEGF-Fc fusion protein including aflibercept.
Owner:ALTEOGEN
Liquid chip kit for detecting lung cancer autoantibody
InactiveCN103383395AImprove detection accuracyRealize joint detectionMaterial analysisBiotin-streptavidin complexAntigen
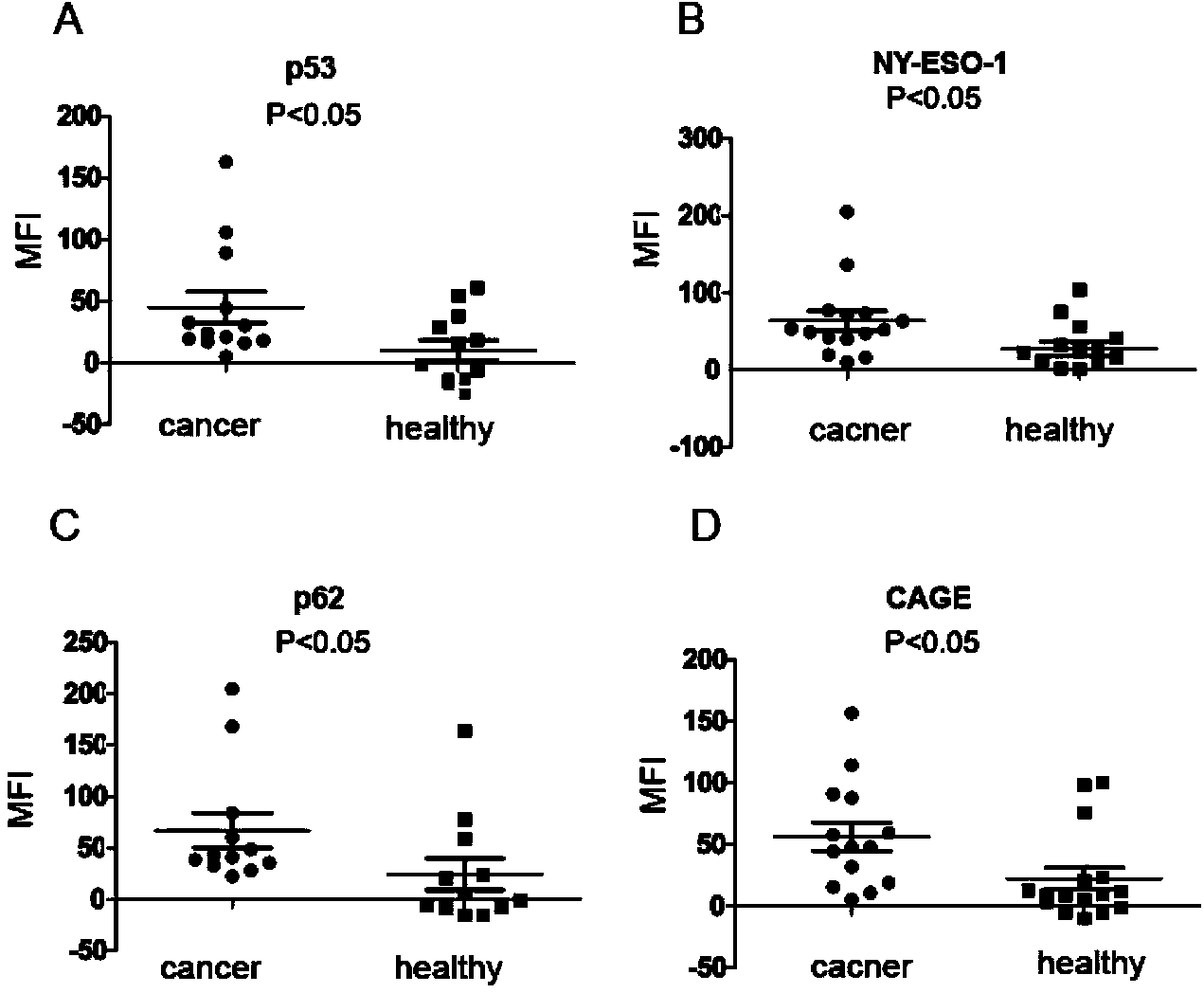
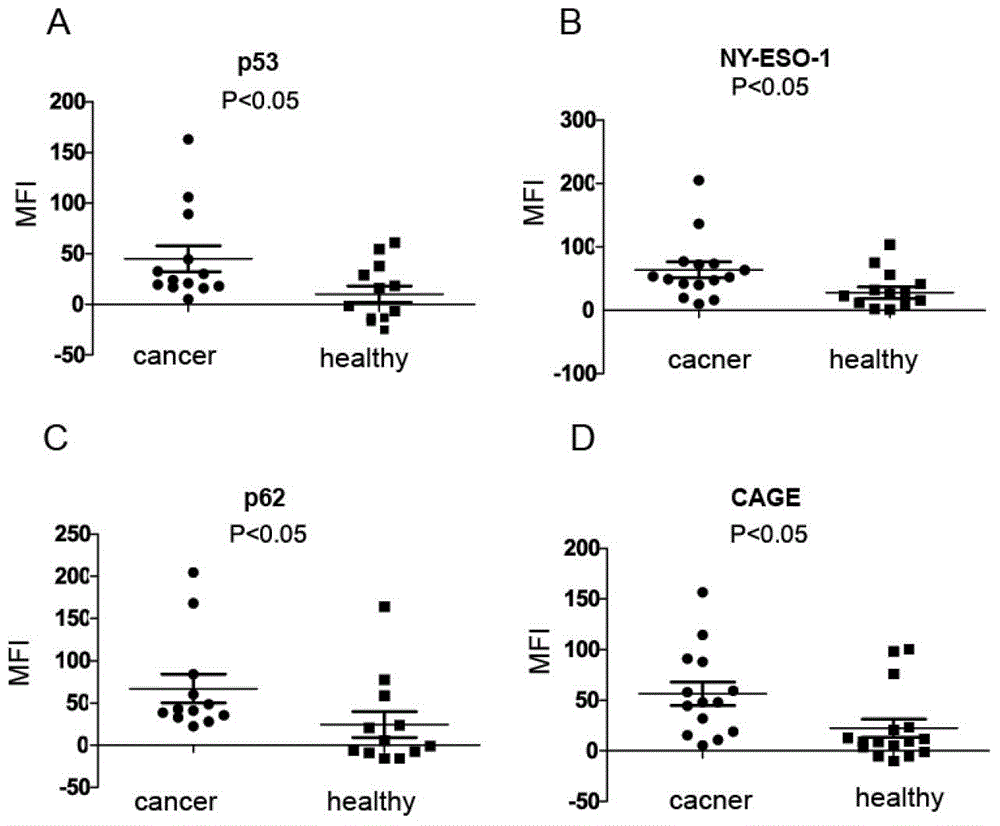
The invention discloses a liquid chip kit for detecting a lung cancer autoantibody. The kit includes: microspheres coupling antigen proteins, a biotin labeled second antibody, streptavidin-phycoerythrin, a reaction buffer and a dilution buffer solution. The antigen proteins are at least two of p62, NY-ESO-1, p53 and CAGE, wherein the microspheres coupling different antigen proteins have different color codes. The second antibody is anti-human immunoglobulin G. The liquid chip kit for detecting the lung cancer autoantibody provided in the invention realizes joint detection of four antibodies in serum on a liquid chip technology platform, and takes the p62 antibody, the NY-ESO-1 antibody, the p53 antibody and the CAGE antibody as marks, has high detection accuracy, and provides a new idea for early diagnosis of lung cancer.
Owner:HANGZHOU FIRST PEOPLES HOSPITAL +2
Novel I-type human immunodeficiency virus (HIV-1) infection enzyme test-free reagent kit
The invention provides a novel I-type human immunodeficiency virus (HIV-1) infection enzyme test-free reagent kit. The reagent kit comprises A, HIV-1gp41 protein, B, a blank enzyme linked plate, C, acid eluent, D, goat anti-human immunoglobulin G (IgG) marked with horseradish peroxidase, E, tetramethyl benzidine (TMB) color development solutions and F, stop solutions.
Owner:BEIJING KINGHAWK PHARMA
Stable liquid formulation of fusion protein with IgG Fc domain
A stable liquid formulation includes a fusion protein having an Fc domain of a human immunoglobulin G (IgG), in particular, a protein in which an Fc domain of a human immunoglobulin G (IgG) and a soluble extracellular domain of a vascular endothelial growth factor (VEGF) receptor are fused (e.g., aflibercept)). A composition for stabilizing a protein and a method for stabilizing a protein in which an Fc domain of an IgG and a soluble extracellular domain of a VEGF receptor are fused are disclosed. The present invention improves therapeutic effects on various ophthalmic diseases (e.g., retinal vein occlusion, diabetic macular edema, choroidal neovascularization and wet age-related macular degeneration, etc.) caused by abnormal angiogenesis, while pursuing stabilization of bioactivity through a stable liquid formulation suitable for intravitreal injection of an anti-VEGF-Fc fusion protein including aflibercept.
Owner:ALTEOGEN
Nano antibody fusion protein, and preparation method and application thereof
InactiveCN103333253AImprove biological activityImprove stabilityAntibody ingredientsPharmaceutical non-active ingredientsAntigenSide effect
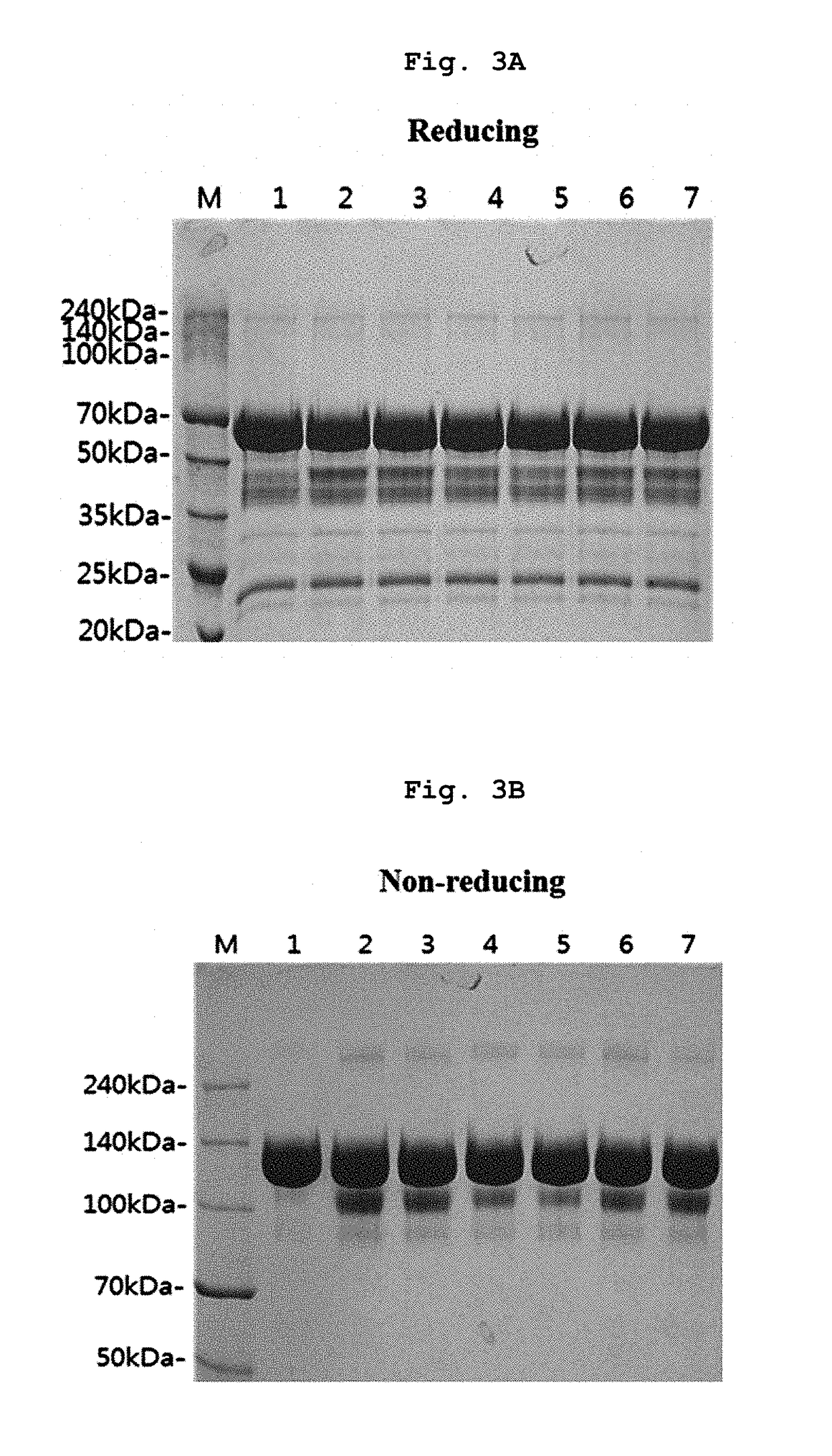
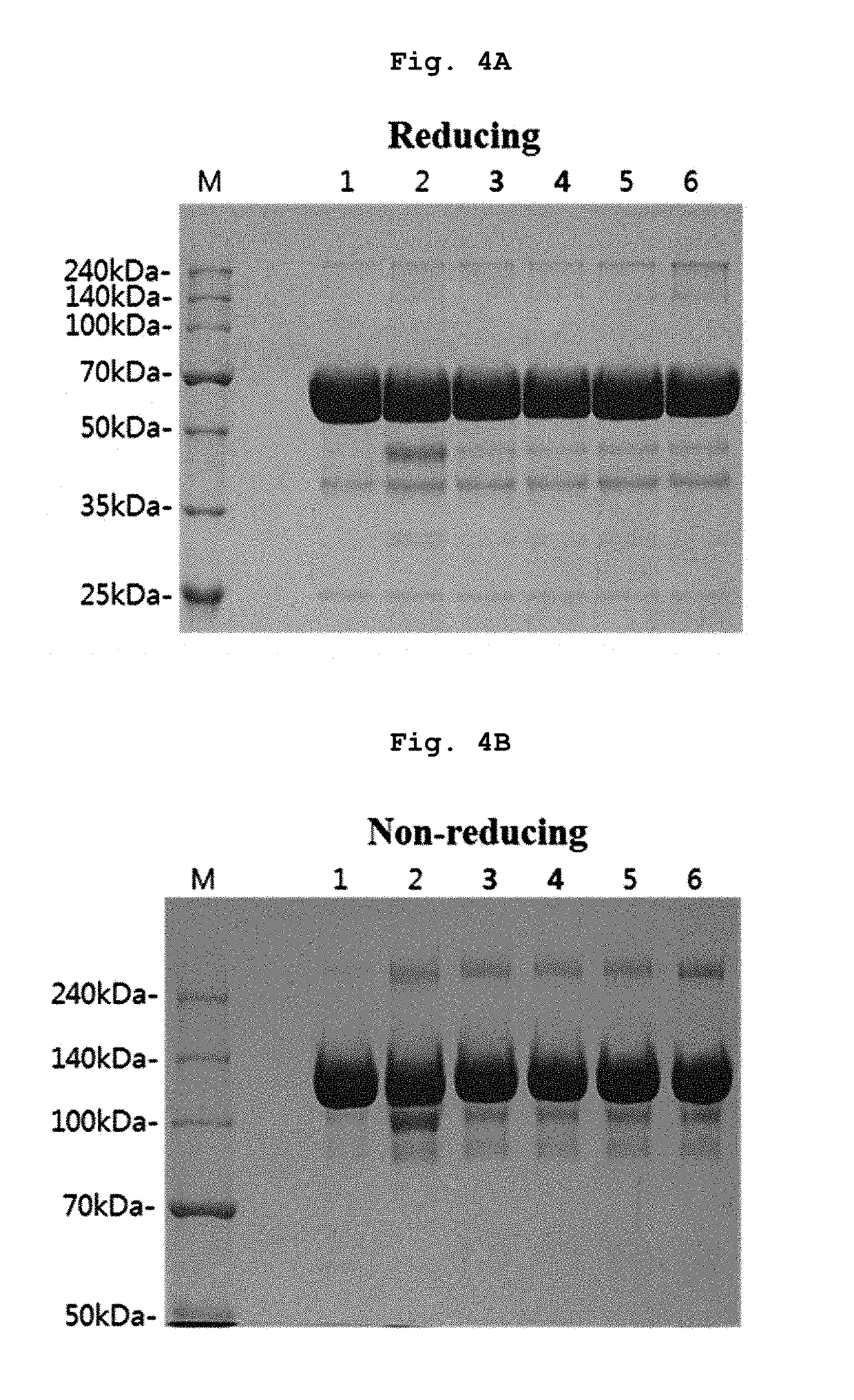
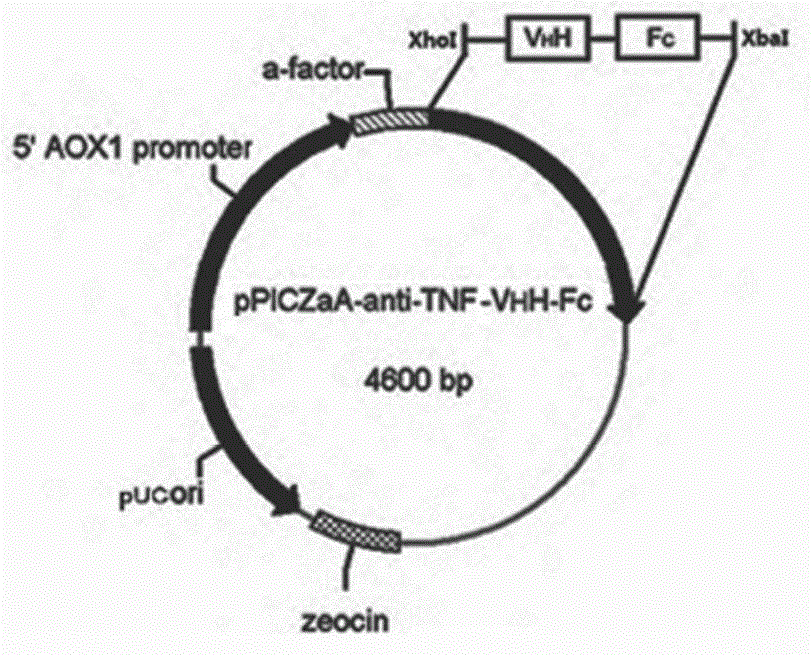
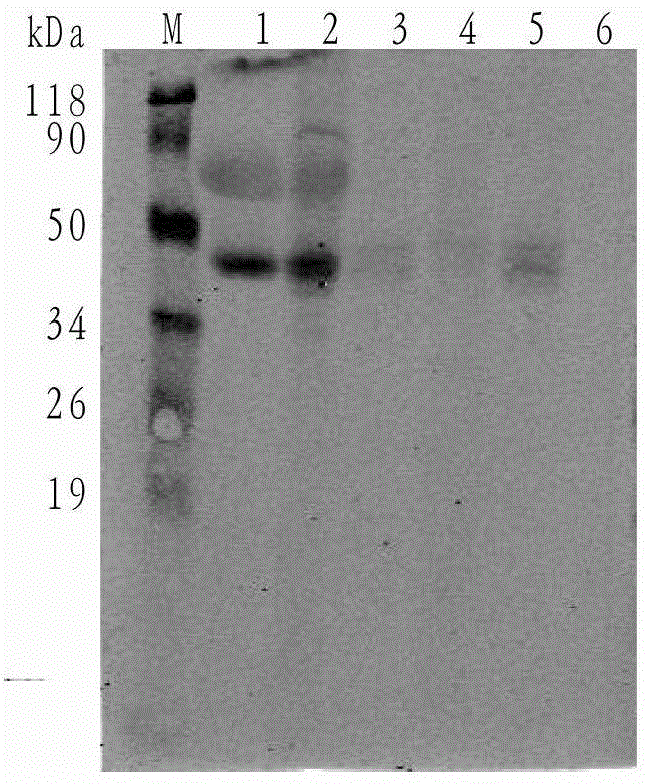
The invention discloses a nano antibody fusion protein which is formed by connecting an Fc segment of a human immunoglobulin G with a VHH antibody segment of camel anti-human TNFa. The invention also discloses a preparation method and application of the fusion protein. Compared with the prokaryotically-expressed TNFa nano antibody and the prokaryotically-expressed Fc-modified TNFa nano antibody, the nano antibody fusion protein disclosed by the invention has higher biological activity; the yeast expression anti-TNF-VHH-Fc protein forms a complete dimer form, and thus, has higher stability and antigen combination activity; the Fc segment of immunoglobulin is selected as a fusion segment, thereby avoiding the generation of side effect; and the invention has the advantages of high expression level and low cost, and can easily implement scale-up production.
Owner:NANJING RUIBIDE BIOTECH
Human urine immunoglobulin G detection kit based on latex-enhanced immunoturbidimetry
InactiveCN109633172AImprove stabilityGood repeatabilityBiological testingHuman immunoglobulinsMicrosphere
The invention, which belongs to the technical field of medical in-vitro diagnostics, relates to a human urine immunoglobulin G detection kit based on latex-enhanced immunoturbidimetry, thereby solvinga problem of providing a human urine immunoglobulin G content detection kit having advantages of wide detection linear range, high detection accuracy, and high detection stability. The kit comprisesan R1 reagent, an R2 reagent and a calibrator. The R1 reagent includes a buffer solution, NaCl and a preservative. The R2 reagent includes a buffer solution, an antibody-coupling latex microsphere, aprotective agent and a preservative; and the antibody is the goat anti-human immunoglobulin polyclonal antibody or a rabbit anti-human immunoglobulin polyclonal antibody. The calibrator is formed by aplurality of solution; each solution includes human immunoglobulin G, a buffer solution and a preservative; and the concentrations of the human immunoglobulin G in different solutions are different.The kit has advantages of wide detection linear range, high detection accuracy, and high detection stability.
Owner:DIRUI MEDICAL TECH CO LTD
Method for detecting concentration of trace immunoglobulin G in human serum
InactiveCN103969446AAccurate, convenient and fast detectionHigh sensitivityBiological testingFluorescence/phosphorescenceReceptorIntravenous gammaglobulin
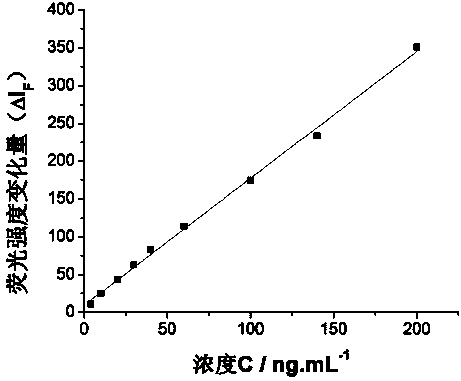
The invention discloses a method for detecting the concentration of trace immunoglobulin G in human serum. An energy transferring system with stable performance consists of goat anti human immunoglobulin G antibody-labeled fluorescein isothiocyanate serving as an energy transferring supplier and nano gold serving as a receptor; the goat anti human immunoglobulin G antibody-labeled fluorescein isothiocyanate transmits energy to the nano gold, so that fluorescence of the fluorescein isothiocyanate is quenched; however, after immunoglobulin G is added, the fluorescence of the fluorescein isothiocyanate is recovered; furthermore, the fluorescence recovery value and the concentration of the immunoglobulin G are in good linear relation within a range of 4.0-220.0 nanograms per milliliter, so that a novel method for detecting the immunoglobulin G is built. According to the method disclosed by the invention, the shortcomings of low sensitivity, complicated operation and the like during detection in the prior art are overcome, and the immunoglobulin G in the human serum can be conveniently and quickly detected.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
A liquid chip kit for detecting autoantibodies in lung cancer
InactiveCN103383395BRealize joint detectionImprove detection accuracyMaterial analysisBiotin-streptavidin complexMicrosphere
The invention discloses a liquid chip kit for detecting a lung cancer autoantibody. The kit includes: microspheres coupling antigen proteins, a biotin labeled second antibody, streptavidin-phycoerythrin, a reaction buffer and a dilution buffer solution. The antigen proteins are at least two of p62, NY-ESO-1, p53 and CAGE, wherein the microspheres coupling different antigen proteins have different color codes. The second antibody is anti-human immunoglobulin G. The liquid chip kit for detecting the lung cancer autoantibody provided in the invention realizes joint detection of four antibodies in serum on a liquid chip technology platform, and takes the p62 antibody, the NY-ESO-1 antibody, the p53 antibody and the CAGE antibody as marks, has high detection accuracy, and provides a new idea for early diagnosis of lung cancer.
Owner:HANGZHOU FIRST PEOPLES HOSPITAL +2
Rabbit antibodies to human immunoglobulins g
PendingUS20210380719A1Immunoglobulins against animals/humansBiological testingHuman immunoglobulinsHuman Immunoglobulin G
This disclosure anti-human IgG antibodies and antigen-binding portions thereof derived from rabbits and methods of using these antibodies and portions.
Owner:GENZYME CORP
Preparation method of redox probe for marking h-IGg (Human-Immunoglobulin G) impedance immunosensor
InactiveCN102914577AEasy to makeLow detection limitBiological testingMaterial resistanceWater bathsBeta-Mercaptoethylamine
The invention discloses a preparation method of a redox probe for marking an h-IGg (Human-Immunoglobulin G) impedance immunosensor. The preparation method comprises the following steps of: dropping an absolute ethyl alcohol solution containing beta-mercaptoethylamine into an absolute ethyl alcohol solution in which 3,5-dibromosalicylaldehyde is dissolved, performing water-bath heating, recrystallizing and drying in vacuum so as to obtain beta-mercaptoethylamine shrinkage 3,5-dibromosalicylaldehyde Schiff base; preparing the absolute ethyl alcohol and N,N-dimethylformamide into a mixture solvent in a volume ratio of 2:1, dropwise adding an N,N-dimethylformamide solution containing nickel acetate into the mixture solvent of the beta-mercaptoethylamine shrinkage 3,5-dibromosalicylaldehyde Schiff base, performing water-bath heating, recrystallizing and drying in vacuum so as to obtain a beta-mercaptoethylamine shrinkage 3,5-dibromosalicylaldehyde Schiff base complex; dissolving the beta-mercaptoethylamine shrinkage 3,5-dibromosalicylaldehyde Schiff base complex into the absolute ethyl alcohol, and further adding into nanogold sol. The redox probe is simple to prepare and has the advantages of low detection limit, good stability, good reproducibility and the like when being used for marking the h-IGg impedance immunosensor to detect the h-IGg content.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Characteristic spectrums of lung cancer and pulmonary nodule proteins as well as construction methods and application of characteristic spectrums
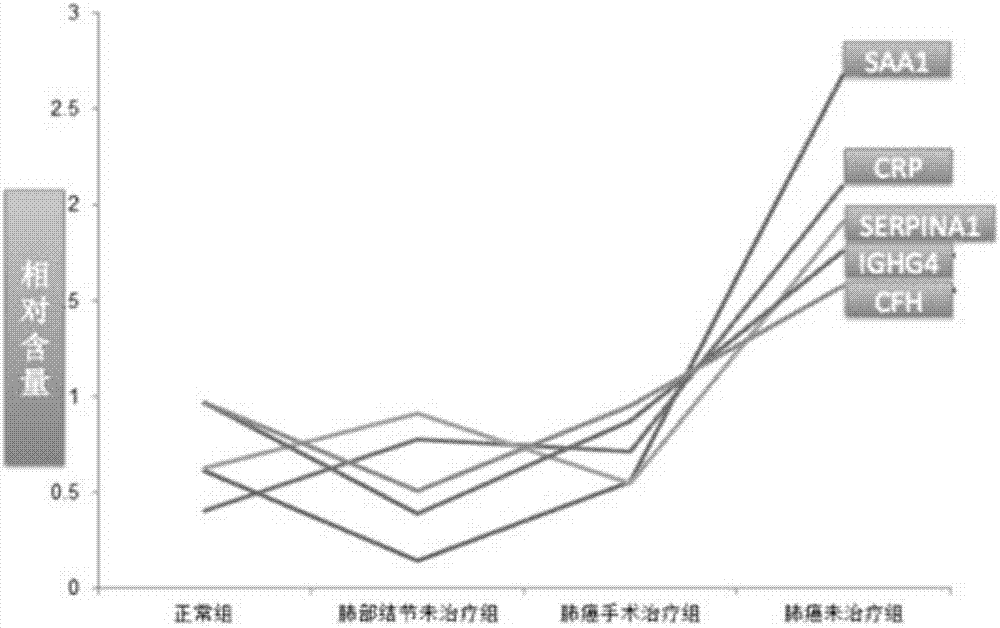
The invention discloses characteristic spectrums of lung cancer and pulmonary nodule proteis as well as construction methods and application of the characteristic spectrums. The methods comprise the following steps of extracting total protein from the plasma of a lung cancer / pulmonary nodule patient group and a healthy control group respectively, carrying out enzymolysis by trypsase, carrying out iTRAQ (isobaric Tags for Relative and Absolute Quantification) labeling, carrying out two-dimensional liquid chromatographic and tandem mass spectrometric detection, and carrying out relative quantitative analysis, so that the characteristic spectrums of the proteins related to the lung cancer / pulmonary nodule are obtained, wherein in the characteristic spectrum of the protein related to the lung cancer, the protein with expression differences in the lung cancer patient group and the healthy control group is up-regulated protein (SAA1 (Serum Amyloid A 1), SERPINA1 (Alpha-1-Antitrypsin), CRP (C-Reactive Protein), IGHG4 (Human Immunoglobulin G 4) and a CFH (Complement Factor H)); in the in the characteristic spectrum of the protein related to the pulmonary nodule, the protein with expression differences in the pulmonary nodule patient group and the healthy control group is up-regulated protein (the SERPINA1 and the CRP) and down-regulated protein (the SAA1, the IGHG4 and the CFH). The characteristic spectrums are used for facilitating the research of the biological natures of the lung cancer and the pulmonary nodule, and have important significance for the normative typing of the lung cancer and the pulmonary nodule.
Owner:北京邦菲生物科技有限公司
Human igg1 derived antibody with pro-apoptotic activity
ActiveUS20160280765A1Increased pro-apoptotic activityPotentialized therapeutic efficiencyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenHeavy chain
A method for increasing the therapeutic efficacy of a human immunoglobulin G class 1 (IgG1) antibody, includes: mutating the human CH1γ1 domain from the antibody, to restore the pairing between CH1 and CL domains that is typical of the other IgG subclasses, or by substituting the human CH1γ1 domain by the CH1 domain from a human IgG2 (CH1γ2), IgG3 (CH1γ3) or IgG4 (CH1γ4); the antibody obtained by such method, includes a) a light chain including the following amino acid sequences: i) the Light Chain Variable Region (LCVR) specific from an antigen; and ii) a human kappa (κ) Constant (CL) domain; and b) a heavy chain including the following amino acid sequences: i) the Heavy Chain Variable Region (HCVR) specific from the antigen; ii) the CH2 and CH3 domains from a human IgG1; and iii) the CH1 domain from a human IgG1, mutated to restore pairing between CH1 and CL domains.
Owner:OGD2 PHARMA
Treatment of alzheimer's disease subpopulations with pooled immunoglobulin g
The present invention provides, among other aspects, methods for the treatment of Alzheimer's disease in a subject in need thereof, the method including administration of a therapeutically effective amount of a pooled human immunoglobulin G (IgG) composition to a subject with moderately severe Alzheimer's disease, a subject carrying an ApoE4 allele, or both, where the amount of pooled human IgG is from 300 mg / kg to 800 mg / kg body weight of the subject per two week period, and where the amount is administered in one or more doses during the two week period after initiation of a therapeutic regimen. Also provided, are methods for selecting a treatment regimen for a subject with Alzheimer's disease, including diagnosing the severity of the Alzheimer's disease, determining if the subject carries an APOE4 allele, or both, and assigning a treatment regimen including administration of pooled human immunoglobulin G and / or an anti-beta amyloid monoclonal antibody.
Owner:TAKEDA PHARMA CO LTD
Procalcitonin detection kit
InactiveCN104714019AReduce dosageLow costBiological testingCoatingsProtein moleculesHorse radish peroxidase
Disclosed is a procalcitonin (PCT) detection kit, which comprises a magnetic particle separation reagent, a PCT detection antibody, an enzyme conjugate, a photogenic substrate, a PCT standard substance, and an enzyme diluent for diluting the enzyme conjugate. The magnetic particle separation reagent contains a magnetic particle coupled protein molecule, which is anti-rat human immunoglobulin G; the PCT detection antibody is an anti-rat IgG antibody; and the enzyme conjugate anti-rat human IgG labeled by horse radish peroxidase. According to the invention, an indirect coating mode that anti-rat human immunoglobulin G is coupled with magnetic particles, and a PCT monoclonal antibody combines with the anti-rat human immunoglobulin G is adopted. Through such mode, the dosage of the PCT monoclonal antibody is lowered, so that cost is reduced. The detection sensitivity is obviously improved by adding the concentration of anti-rat human immunoglobulin G of coupled magnetic particles.
Owner:上海国兴医疗器械有限公司
Preparation method of anti-pollution electrochemical biosensor based on temperature-sensitive western blot gel
ActiveCN112595765AStrong anti-pollutionAbility to eliminate non-specific adsorptionMaterial electrochemical variablesAgainst vector-borne diseasesElectrochemical biosensorO-Phosphoric Acid
Owner:QILU UNIV OF TECH
Cd80 extracellular domain fc fusion protein dosing regimens
PendingCN112543642AOrganic active ingredientsCell receptors/surface-antigens/surface-determinantsExtracellularCD80
The present disclosure provides methods of administering fusion proteins comprising the extracellular domain of human cluster of differentiation 80 (CD80) and the fragment crystallizable (Fc) domain of human immunoglobulin G 1 (IgG1) to a subject in need thereof, for example, a cancer patient.
Owner:FIVE PRIME THERAPEUTICS
Concentrated human immunoglobulin composition
InactiveUS9186401B2Good flexibilityImprove the quality of lifeAntibacterial agentsNervous disorderHuman Immunoglobulin GImmunoglobulin Fc
The invention relates to a human immunoglobulin G composition characterized in that the human immunoglobulin G concentration is at least 230 g / l, which is of use in particular for subcutaneous administration.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Human IgG1 derived antibody with pro-apoptotic activity
ActiveUS10669328B2High activityEffective therapyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHuman immunoglobulinsHeavy chain
A method for increasing the therapeutic efficacy of a human immunoglobulin G class 1 (IgG1) antibody, includes: mutating the human CH1γ1 domain from the antibody, to restore the pairing between CH1 and CL domains that is typical of the other IgG subclasses, or by substituting the human CH1γ1 domain by the CH1 domain from a human IgG2 (CH1γ2), IgG3 (CH1γ3) or IgG4 (CH1γ4); the antibody obtained by such method, includes a) a light chain including the following amino acid sequences: i) the Light Chain Variable Region (LCVR) specific from an antigen; and ii) a human kappa (κ)Constant (CL) domain; and b) a heavy chain including the following amino acid sequences: i) the Heavy Chain Variable Region (HCVR) specific from the antigen; ii) the CH2 and CH3 domains from a human IgG1; and iii) the CH1 domain from a human IgG1, mutated to restore pairing between CHI and CL domains.
Owner:OGD2 PHARMA
Preparation method of redox probe for marking h-IGg (Human-Immunoglobulin G) impedance immunosensor
InactiveCN102914577BEasy to makeLow detection limitBiological testingMaterial resistanceWater bathsBeta-Mercaptoethylamine
The invention discloses a preparation method of a redox probe for marking an h-IGg (Human-Immunoglobulin G) impedance immunosensor. The preparation method comprises the following steps of: dropping an absolute ethyl alcohol solution containing beta-mercaptoethylamine into an absolute ethyl alcohol solution in which 3,5-dibromosalicylaldehyde is dissolved, performing water-bath heating, recrystallizing and drying in vacuum so as to obtain beta-mercaptoethylamine shrinkage 3,5-dibromosalicylaldehyde Schiff base; preparing the absolute ethyl alcohol and N,N-dimethylformamide into a mixture solvent in a volume ratio of 2:1, dropwise adding an N,N-dimethylformamide solution containing nickel acetate into the mixture solvent of the beta-mercaptoethylamine shrinkage 3,5-dibromosalicylaldehyde Schiff base, performing water-bath heating, recrystallizing and drying in vacuum so as to obtain a beta-mercaptoethylamine shrinkage 3,5-dibromosalicylaldehyde Schiff base complex; dissolving the beta-mercaptoethylamine shrinkage 3,5-dibromosalicylaldehyde Schiff base complex into the absolute ethyl alcohol, and further adding into nanogold sol. The redox probe is simple to prepare and has the advantages of low detection limit, good stability, good reproducibility and the like when being used for marking the h-IGg impedance immunosensor to detect the h-IGg content.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Molecularly imprinted electrochemical sensor based on electro-copper-based mofs sensitive membrane modified electrode and preparation method and detection method thereof
ActiveCN112903781BHighly selective adsorptionFast electrical signal responseMaterial electrochemical variablesProtein targetIntravenous gammaglobulin
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Treatment of alzheimer's disease subpopulations with pooled immunoglobulin g
InactiveUS20190144530A1Delay progressNervous disorderPeptide/protein ingredientsAPOE4 AlleleMonoclonal antibody
Owner:TAKEDA PHARMA CO LTD
Method for producing human monoclonal immunoglobuling antibodies
The present invention a method for producing a human immunoglobulin G (IgG) antibody using a prime-boost regime in a Bone Marrow Liver Thymic (BLT) mouse.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
The prognosis prediction model can be used for purifying hydrophobic cyclic peptide ligands of human immunoglobulin G
PendingCN112466390AEasy way to handle cyclic peptidesAccurate processingMolecular designSequence analysisCyclic peptidePeptide ligand
The invention provides a hydrophobic cyclic peptide ligand capable of being used for separating and purifying human immunoglobulin G. The hydrophobic cyclic peptide ligand is obtained through a computer simulation bionic design method, and the bionic design method takes an amino acid sequence Ser383-Asn389 on a CH3 binding region on a SpA and hIgG-Fc fragment as a simulation system. Hydrophobic cyclic peptide ligands which can be specifically combined with hIgG are determined through LeDock molecular docking, FlexX molecular docking and Amber molecular dynamics simulation screening, and the cyclic peptide ligand chromatographic medium is adopted to perform purification research on hIgG from a human serum sample, so that purity as high as 96% and recovery rate as high as 92% can be obtained; the hydrophobic cyclic peptide ligand sequence is -[HWG-C-AKTE]-, -[YF-C-WRHE]-, [-WV-C-LHHYF]-, -[PYF-C-TIE]- and -[FY-C-DEHL]-, wherein the peptide ligand with the best effect is -[HWG-C-AKTE]-.
Owner:钮雪琴
A kit for detecting immunoglobulin g in urine
ActiveCN113156136BHigh sensitivityWide linear rangeDisease diagnosisBiological testingActive agentPolyethylene glycol
The application discloses a kit for detecting immunoglobulin G in urine, which belongs to the technical field of medical in vitro diagnosis. The kit includes reagent R1 and reagent R2; the reagent R1 includes the following components: Tris base, polyethylene glycol, polyvinylpyrrolidone, sodium chloride, surfactant S9, EDTA-Na 2 and sodium benzoate; the reagent R2 includes the following components: tris base, goat anti-human immunoglobulin G polyclonal antibody, animal serum, sodium benzoate, trehalose, EDTA-Na 2 and Twain. The kit of this application has high sensitivity, wide linear range and good repeatability, and can accurately detect low-value samples; at the same time, the kit of this application also has good stability, and the reagents can be stably stored in 14 samples It eliminates the filtration step before the reagent is used, which simplifies the operation steps and improves the accuracy of the test results.
Owner:BEIJING DIAGREAT BIOTECH CO LTD
A nanobody fusion protein and its preparation method and application
InactiveCN103333253BImprove biological activityImprove stabilityAntibody ingredientsPharmaceutical non-active ingredientsSide effectProkaryotic expression
The invention discloses a nano antibody fusion protein which is formed by connecting an Fc segment of a human immunoglobulin G with a VHH antibody segment of camel anti-human TNFa. The invention also discloses a preparation method and application of the fusion protein. Compared with the prokaryotically-expressed TNFa nano antibody and the prokaryotically-expressed Fc-modified TNFa nano antibody, the nano antibody fusion protein disclosed by the invention has higher biological activity; the yeast expression anti-TNF-VHH-Fc protein forms a complete dimer form, and thus, has higher stability and antigen combination activity; the Fc segment of immunoglobulin is selected as a fusion segment, thereby avoiding the generation of side effect; and the invention has the advantages of high expression level and low cost, and can easily implement scale-up production.
Owner:NANJING RUIBIDE BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com