Recombinant oxyntomodulin (OXM) fusion protein, and preparation and application thereof
An oxyntomodulin and fusion protein technology, applied in the field of treatment of metabolic diseases such as diabetes and obesity, can solve problems such as poor in vivo stability, and achieve the effects of good stability, easy to scale up production and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Obtaining OXM target gene and human IgG1 Fc fragment gene
[0051] According to the OXM gene sequence, its amino acid sequence is SEQ ID NO: 1, and its gene sequence is SEQ ID NO: 2, which was submitted to Gene Synthesis Company for synthesis. According to the human IgG1 Fc gene sequence, using normal human lymphocyte total RNA as a template, RT-PCR amplifies the IgG1 Fc fragment. The reaction conditions are as follows: After the RT-PCR reaction mixture is denatured at 50°C for 30 minutes, the reaction is carried out according to the following conditions: reverse Recording reaction: denaturation at 94°C for 30 seconds; annealing at 55°C for 30 seconds; extension at 68°C for 1 minute, 10 cycles of reaction. PCR reaction: Denaturation at 94°C for 30 seconds; annealing at 60°C for 30 seconds; extension at 68°C for 1 minute, 25 cycles of reaction. It was then extended for an additional 12 minutes at 68°C. After the reaction was completed, RT-PCR products were...
Embodiment 2
[0052] Embodiment 2: Construction of recombinant plasmid
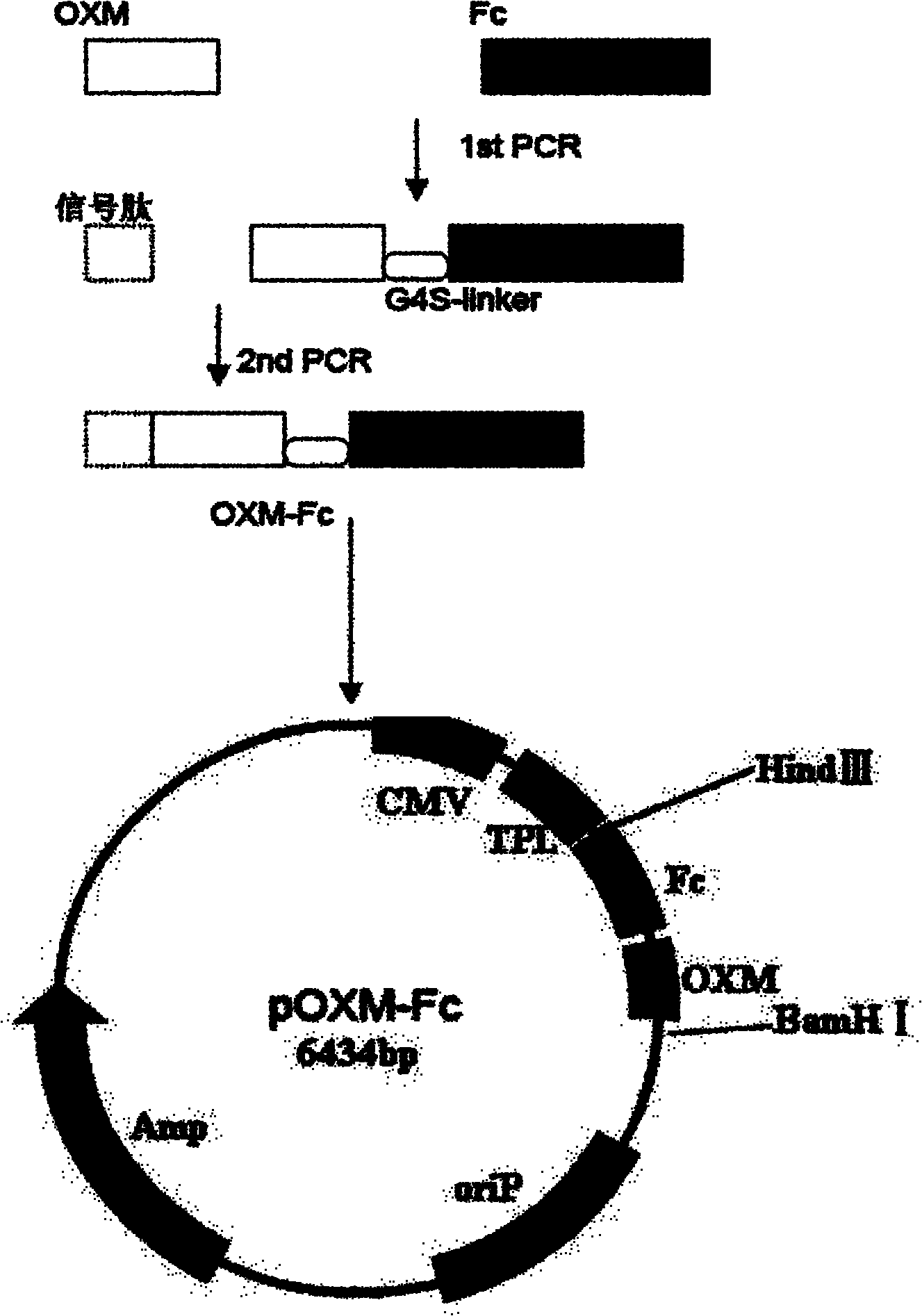
[0053] The vector is constructed as figure 1 As shown, the OXM and Fc gene sequences were assembled by over-lap PCR technology; the mouse Igκ signal peptide was connected to the amino terminal of the OXM-Fc sequence by three PCRs using primer extension PCR technology; and the target gene was combined with pcDNA3.0 The plasmids were digested with Hind III and EcoR V endonucleases respectively; the digested products were ligated with T4 ligase, and the constructed plasmid was named pcDNA3.0 / OXM-Fc. specifically is:
[0054] 1.1 Design the following primers according to the OXM and human IgG1 Fc (hinge+ CH2+ CH3) cDNA sequences:
[0055] POXM1: 5'CATGGCGAGGGCACCTTCACCTC 3'
[0056] POXM2: 5' CAGGTGTGGGTCTTGTCAGAGGAC TTGGGC3'
[0057] PFc1: 5' GTCCTCTGACAAGACCCACACCTG 3'
[0058] PFc2: 5' CGCGGATCC TCACTTGCCGGGGCTCAGGGACAG3'
[0059] Among them, the italic part of POXM2 and PFc1 is the complementary region,...
Embodiment 3
[0065] Example 3: Screening and Identification of CHO Stable Expression Strains (CHO-OXM Cells)
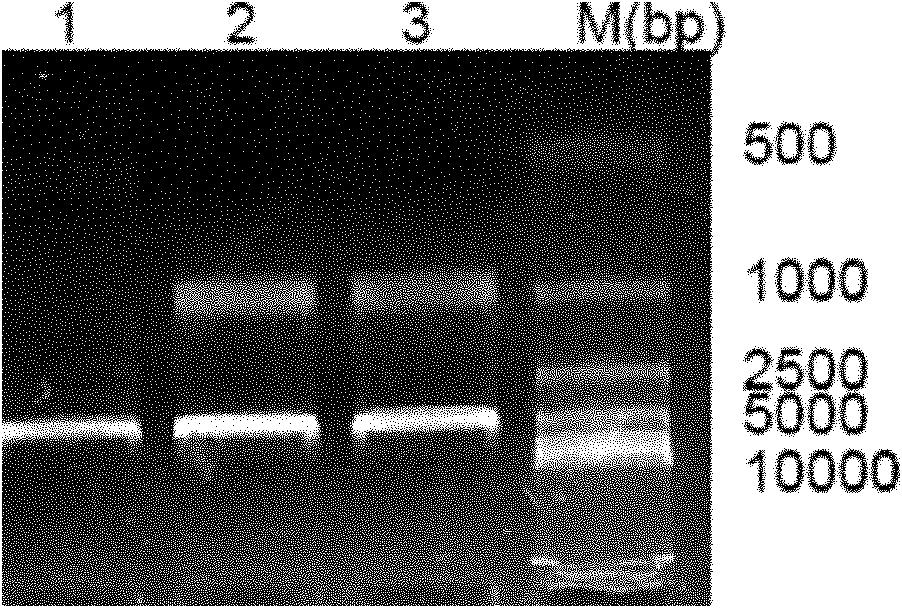
[0066] 1) Take 10 ug of the pOXM-Fc plasmid prepared in Example 2 in large quantities, and use liposome Lipofectin2000 to transfect CHO cells. Two days later, passaging at a ratio of 1:5, adding 0.4 mg / ml G418 for selection, clonal formation can be seen in 10 days. Randomly digest 50 single clones with clear margins and good cell state and inoculate them in 24-well plate culture (the first round of screening).
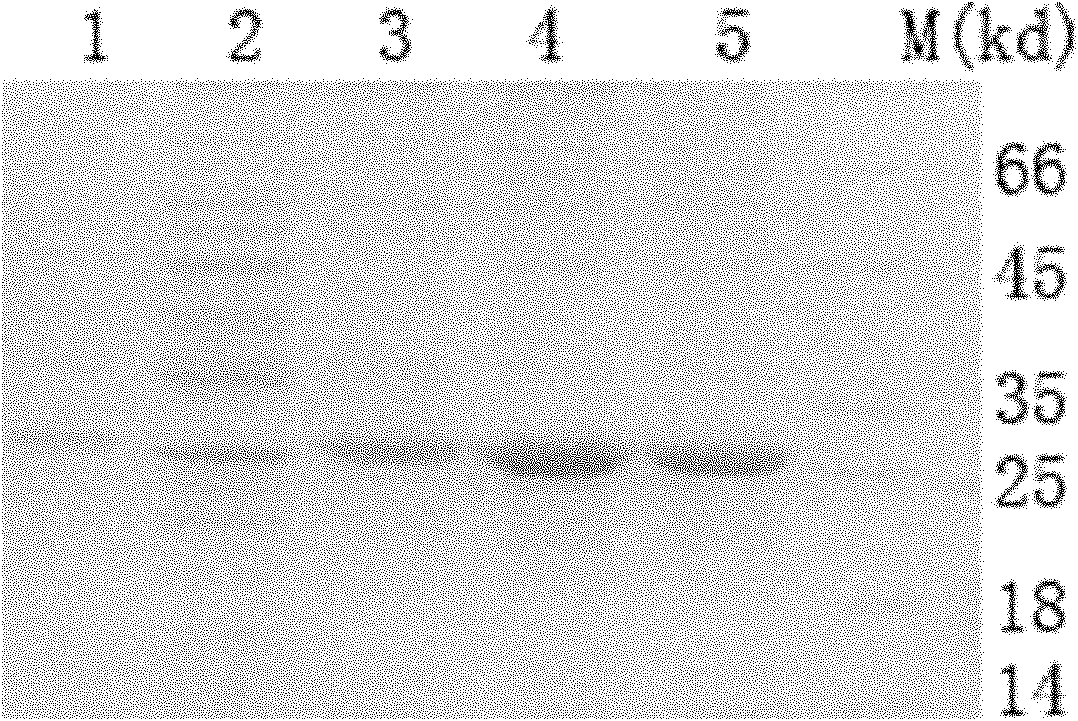
[0067] 2) Please use ELISA to detect the expression of the fusion protein on the three-day culture, and select 15 clones with positive expression and inoculate them in 24-well plates (second round of screening) and 6-well plates (for seed preservation). After 4 days of culture, the supernatant was taken to detect the expression of the fusion protein by ELISA, and four clones with higher expression were selected: A5-3, B2-3, B2-5, and C1-4 were screened by limiting dilut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com