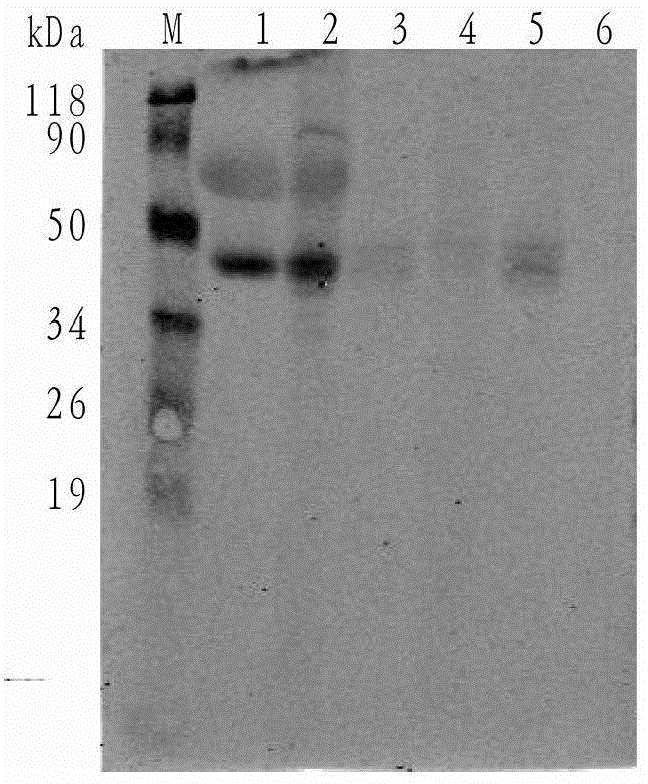
A nanobody fusion protein and its preparation method and application
A fusion protein and nanobody technology, applied in the direction of antibodies, chemical instruments and methods, hybrid peptides, etc., can solve the problem of expensive TNFa antagonists, achieve high stability and antigen binding activity, easy to scale up production, and avoid side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Acquisition of fusion gene anti-TNF-VHH-Fc
[0034] 1. PCR reaction to obtain anti-TNF-VHH gene:
[0035] Set up the following reaction system in a 25μl PCR reaction tube:
[0036]
[0037] The reaction conditions are as follows:
[0038]
[0039] anti-TNF-VHH-F is the upstream primer of anti-TNF-VHH gene, its nucleotide sequence is: CCGGCTCGAGAAAAGAGAGGCTCAGGTGCAGCTGGTGGAGT
[0040] anti-TNF-VHH-R is the downstream primer of anti-TNF-VHH gene, nucleotide sequence TTTGTCACAAGATTTGGGCTCAGAGGAGACGGTGACTTG
[0041] 2. PCR reaction to obtain Fc gene:
[0042] The reaction system is as follows:
[0043]
[0044] The reaction conditions are as follows:
[0045]
[0046] Fc-F is the upstream primer of the Fc gene, its nucleotide sequence is CAAGTCACCGTCTCCTCTGAGCCCAAATCTTGTGACAAA;
[0047] Fc-R is the downstream primer of the Fc gene, and its nucleotide sequence is GCCTCTAGATCATTTACCCGGAGACAGGGA.
[0048] 3. Connect anti-TNF-VHH and human IgG1 Fc gene, and p...
Embodiment 2
[0056] Construction of pPICZa-anti-TNF-VHH-Fc / GS115 eukaryotic expression strain
[0057] 1. After the PCR product is separated by electrophoresis, cut the gel to recover the target fragment, and establish the following enzyme digestion reaction system with the carrier:
[0058]
[0059] React at 37°C for 4h.
[0060] 2. Without purifying the digested mixture, directly phosphorylate the target gene and dephosphorylate the carrier, the steps are as follows.
[0061] Phosphorylation system (Takara reagent):
[0062]
[0063] Dephosphorylation system (Takara reagent):
[0064]
[0065] Then establish the following connection reaction system:
[0066]
[0067] Reaction at 16°C for 12h.
[0068] After double digestion, the anti-TNF-VHH-Fc gene was connected to the yeast expression vector pPICZaA vector, and the successfully constructed yeast expression vector pPICZaA-anti-TNF-VHH-Fc was obtained. The vector construction diagram is shown in figure 2 .
Embodiment 3
[0070] Construction of pPICZa-anti-TNF-VHH-Fc / GS115 eukaryotic expression strain
[0071] 1. Linearization of plasmids:
[0072] The sequenced correct recombinant plasmid pPICZaA-anti-TNF-VHH-Fc / control plasmid pPICZaA was digested with Sac?.
[0073] The reaction system is as follows:
[0074]
[0075] 10 parallel experimental systems, a total of 200uL, reaction conditions: 37°C, 5h
[0076] 2. Purification, concentration and identification of linearized plasmids:
[0077] Combine the enzyme digestion reaction solution of the plasmid into a sterilized 1.5mL EP tube, add 3 times the volume of absolute ethanol, and mix with 1 / 3 volume of 3M NaAc; stand at -70°C for 30min to precipitate DNA; 4°C , Centrifuge at 12000rpm for 30min, discard the supernatant, place at room temperature, wait for ethanol to volatilize, add 10uL sterilized double distilled water to dissolve, and store at -20℃ for later use.
[0078] 3. Transformation of yeast:
[0079] (1) Preparation of yeast ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com