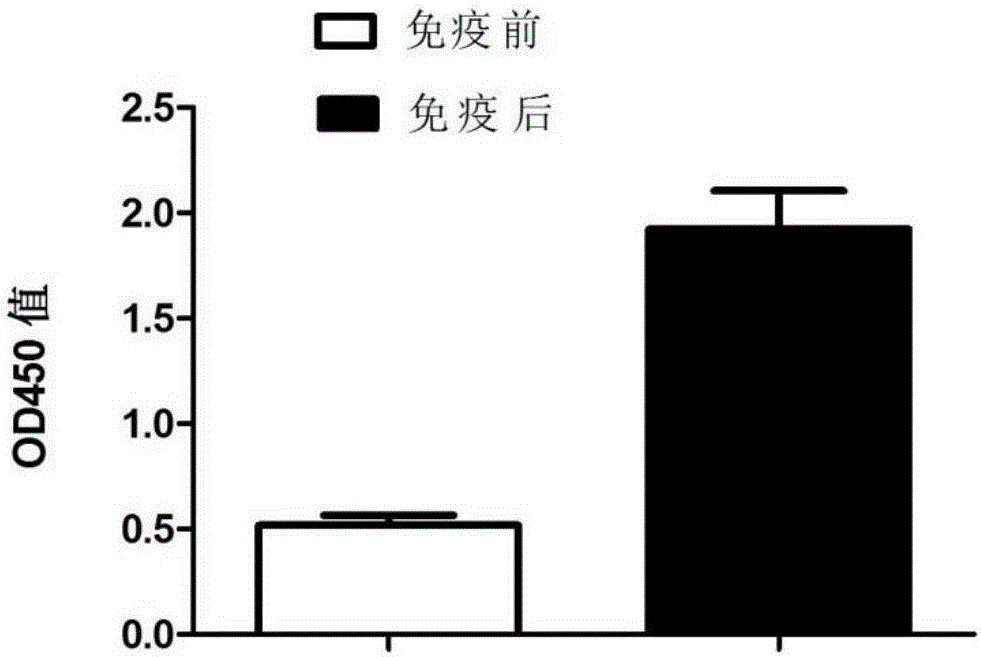
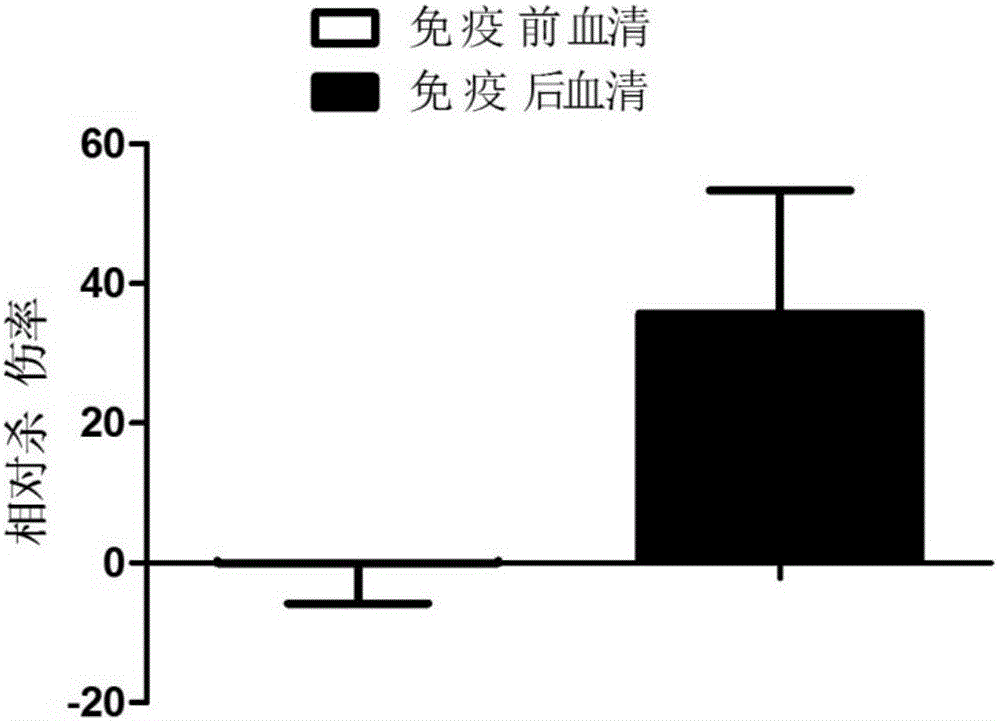
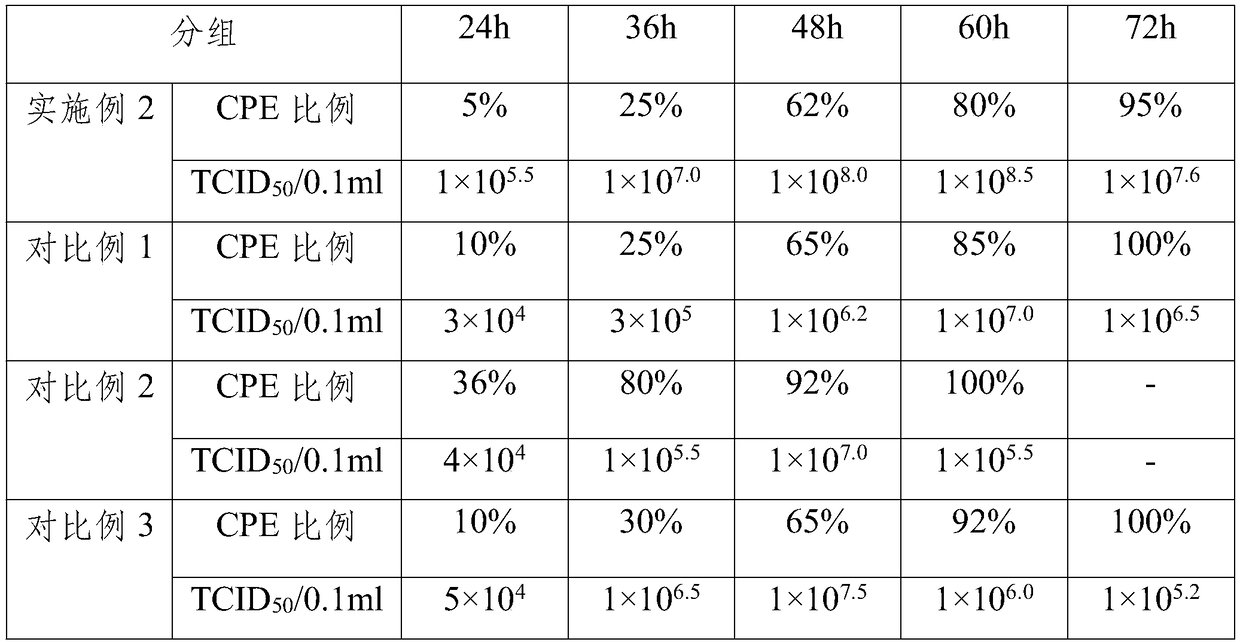
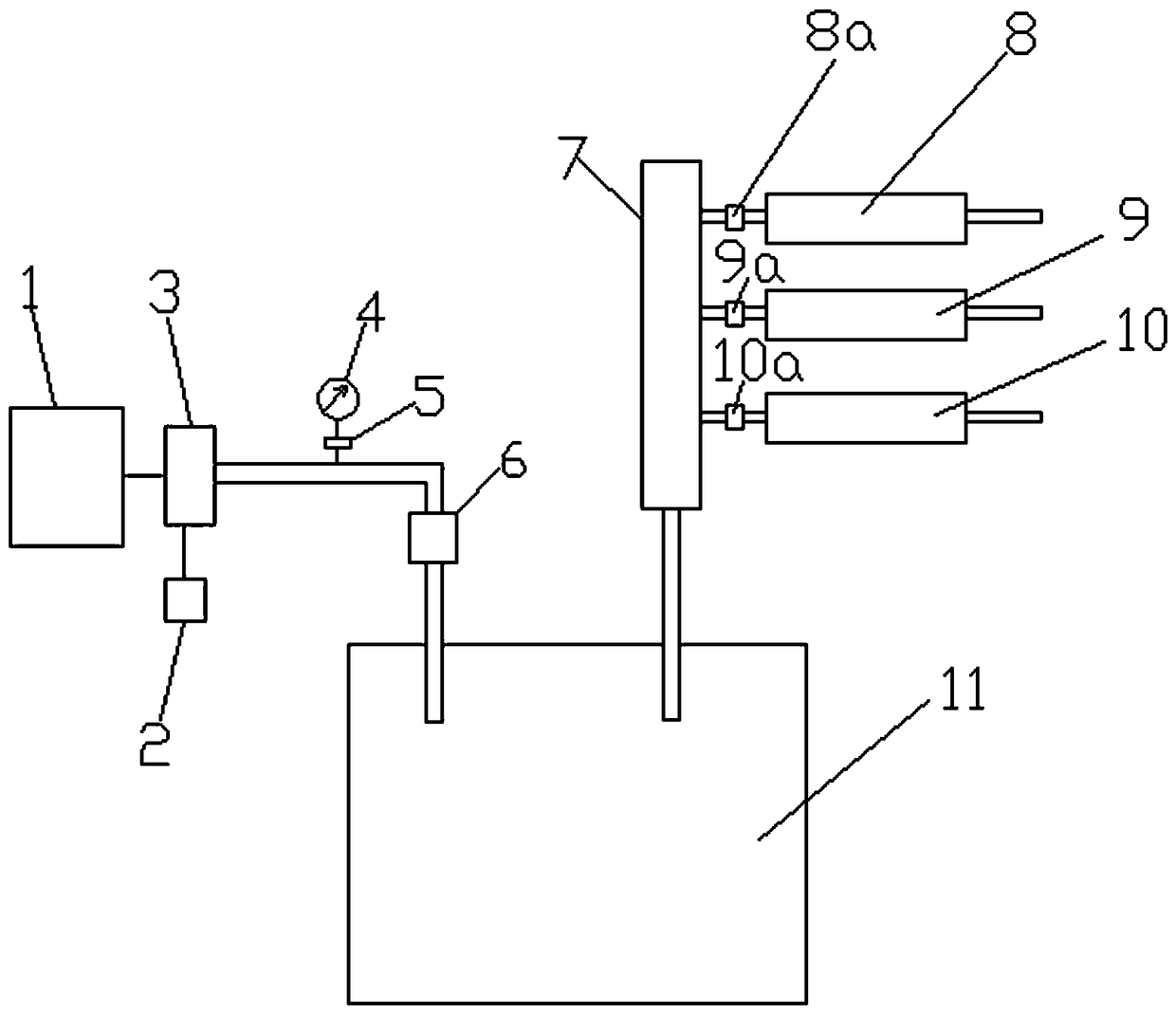
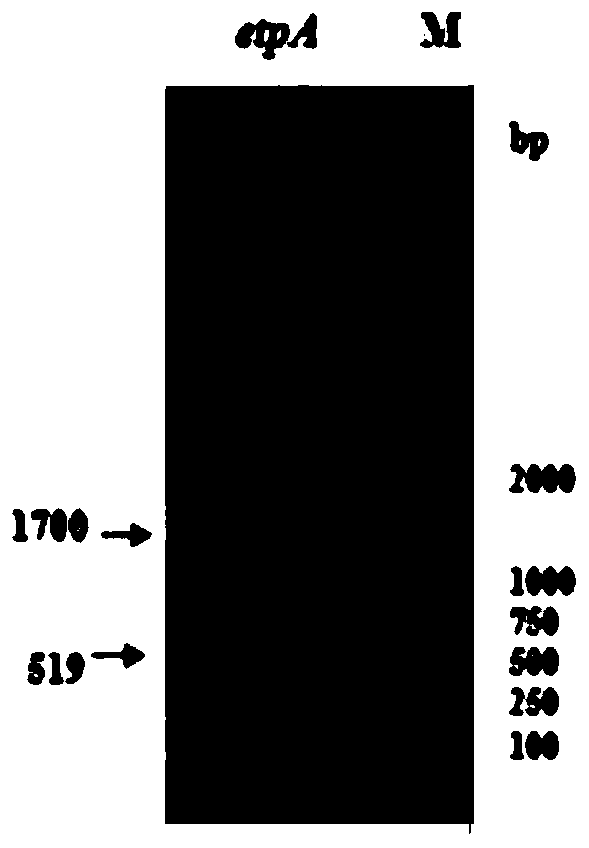Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Challenge tests" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sterilization indicator test packs
InactiveUS7045343B2Improve the immunityBioreactor/fermenter combinationsBiological substance pretreatmentsDefining lengthChallenge tests
Sterilization indicator test packs for testing the effectiveness of a sterilization procedure are provided. Both non-challenge and challenge-type test packs are disclosed. The non-challenge test packs include generally comprise a tray for holding a sterilization indicator the tray containing an elevated rim surface defining the perimeter of the tray and a recessed trough for receiving a sterilization indicator. The rim surface includes a plurality of spaced-apart grooves along its length extending through the rim to the recessed trough; a sterilization indicator within the recessed trough of the tray; and a lid that forms a plurality of channels with the grooves in the tray such that sterilant can enter the tray through the channels and contact the sterilization indicator.The challenge-type test packs generally include a tray for holding a sterilization indicator, the tray comprising a substantially planar surface, a recessed trough for receiving a sterilization indicator, and a recessed groove of a defined length and cross-sectional area extending through the recessed trough and penetrating the edge of the tray; a sterilization indicator within the recessed trough of the tray; and a lid forming a substantially sterilant-impermeable seal with the planar surface of the tray and forming a lumen path with the recessed groove in the tray.
Owner:3M INNOVATIVE PROPERTIES CO
Sterilization indicator with chemically stabilized enzyme
InactiveUS6897059B2Improve the immunityBioreactor/fermenter combinationsBiological substance pretreatmentsAlcoholPlasma sterilization
A sterilization indicator for testing the effectiveness of a sterilization procedure comprises a source of an enzyme, a sterilant-resistant chemical associated with the enzyme, and a substrate that reacts with the enzyme to form a detectable enzyme-modified product that provides an indication of the failure of the sterilization procedure. The sterilant-resistant chemical may be a polyglycerol alkyl ester, polyglycerol alkyl ether, an ethoxylated polyhydric alcohol ester, or a polyhydric alcohol ether. The indicator may be used to test the effectiveness of a hydrogen peroxide plasma sterilization procedure and may be provided with a non-challenge test pack or a lumen-challenge test pack.
Owner:3M INNOVATIVE PROPERTIES CO
In-tank antiseptic antimildew agent composition integrated with dry film for paint
ActiveCN102702839AReduce dosageLow toxicityBiocidePaints with biocidesWater basedMicrobial challenge
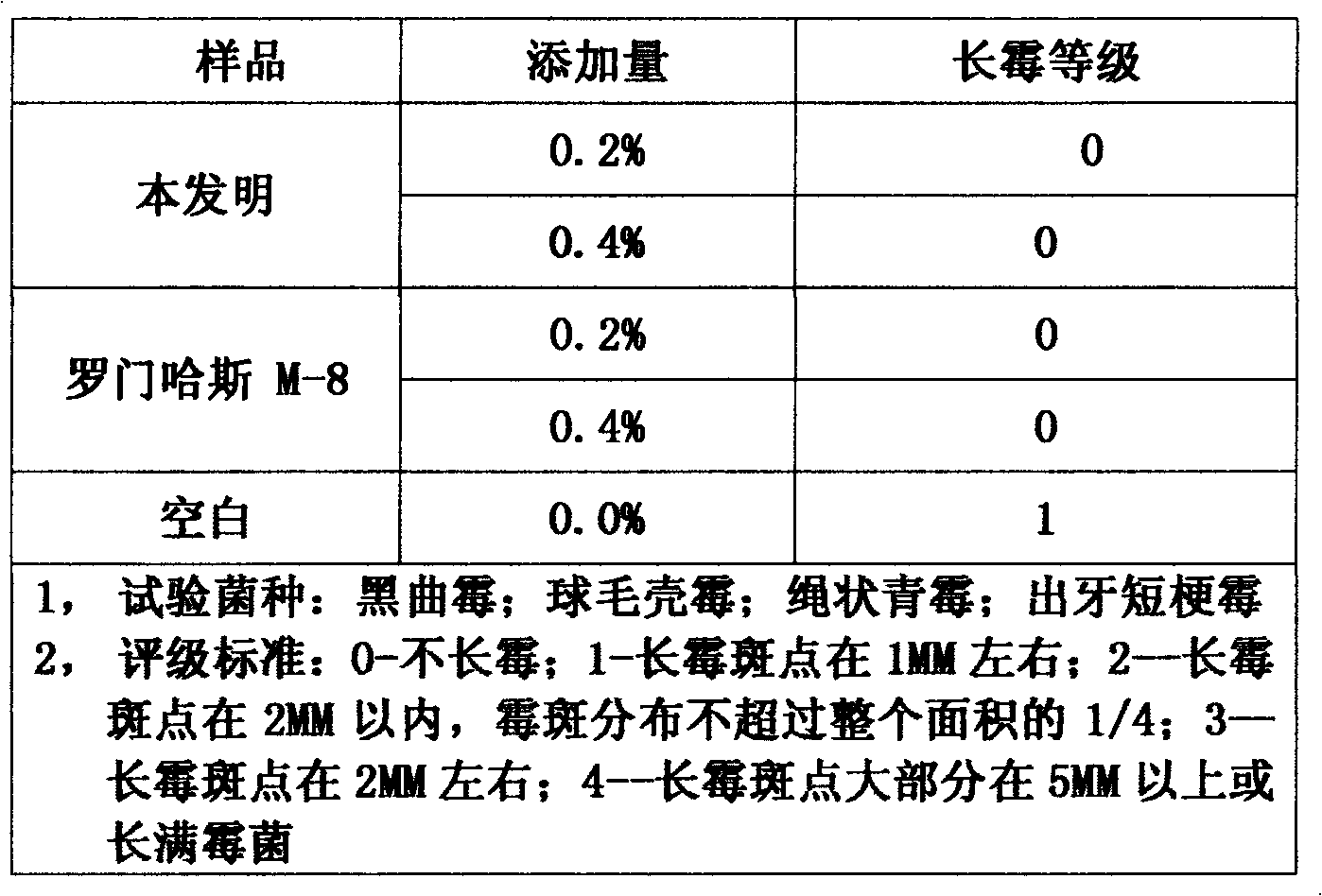
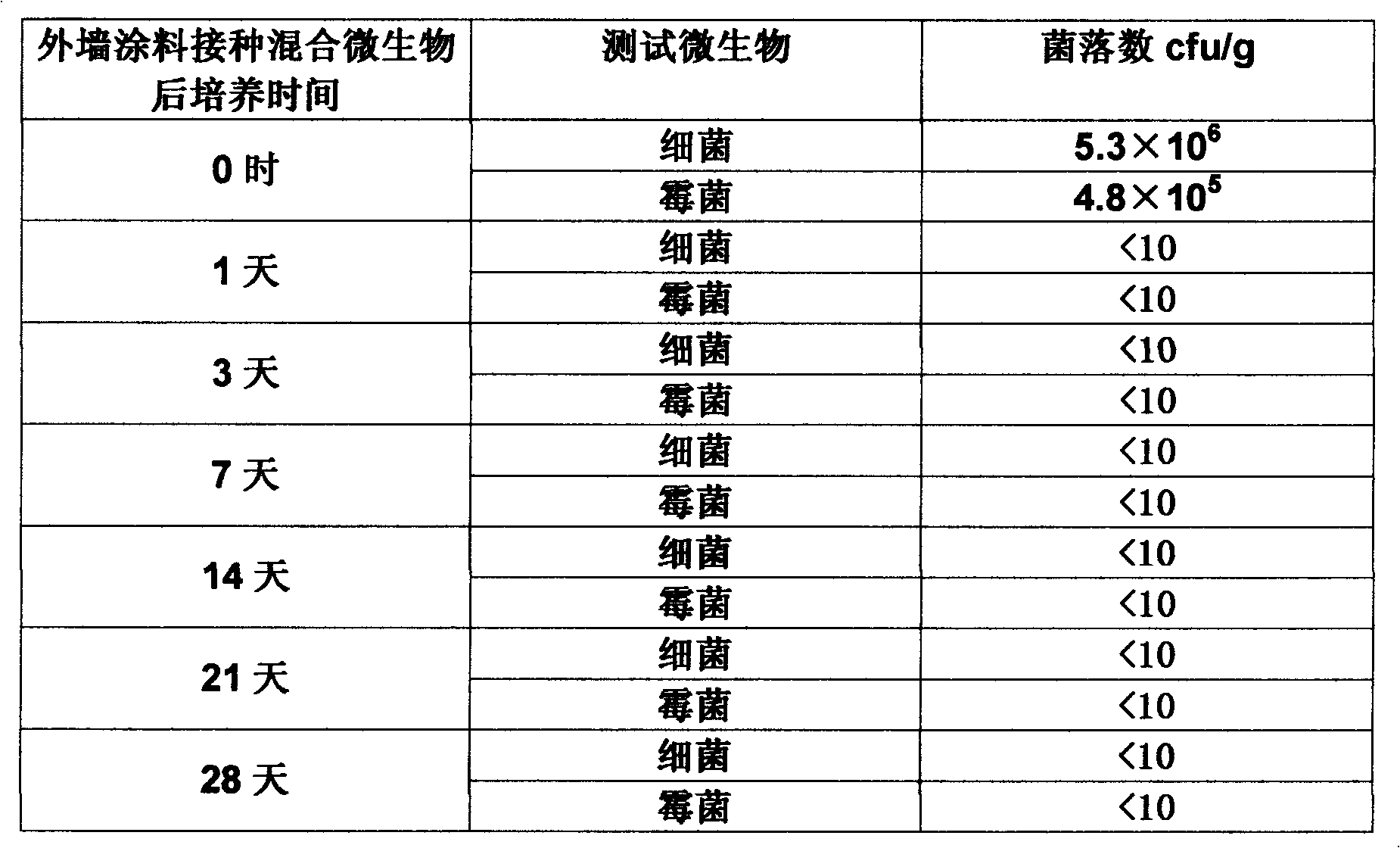
The invention relates to an in-tank antiseptic antimildew agent composition integrated with a dry film for paint. The composition is prepared from a water-based paint in-tank antiseptic bactericide, a dry film antimildew agent, a solvent, an aid, a stabilizing agent and water. The in-tank antiseptic antimildew agent composition integrated with a dry film for paint has the advantages of reasonable formula, advanced process, good corrosion and mildew prevention effects, good stability, and the like. When the in-tank antiseptic antimildew agent composition integrated with a dry film for paint is used for paint, salt cluster phenomena can be avoided, an in-tank antiseptic effect can pass a microorganism challenge test, and the dry film mildew-growing grade is a zero grade.
Owner:SHAANXI RES DESIGN INST OF PETROLEUM CHEM IND
Riemerella anatipestifer blood serum 1 type genetic engineering attenuated strain and construction method thereof
InactiveCN101381695AReduce chanceReduced toxicityBacteriaMicroorganism based processesGenetic engineeringChallenge tests
The invention discloses an attenuated bacterial strain of Riemerella anatipestifer serum type 1 genetic engineering and a method for constructing the same, the method comprises the steps of: performing Sal I single enzyme digestion to a suicide plasmid pDS-132 and a Riemerella anatipestifer serotype 1 gene delta AcOAT, respectively, and using T4DNA ligase for connection to obtain pDS132::delta AcOAT suicide plasmid; transferring pDS-132:: delta AcOAT into a Riemerella anatipestifer serotype 1 gene GDGZ strain competent cell by electrotransformation, and screening recombinant mutant strains with a chloramphenicol resistant plate to obtain the attenuated strain of Riemerella anatipestifer serum type 1 genetic engineering. Animal tests show that 10-time LD50 dosage challenge tests show that the virulence of the attenuated strain has decreased remarkably without virulence basically and the attenuated strain can not reverse to virulence from avirulence and has the potential for preparing attenuated live vaccine.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Classical swine fever E2 subunit vaccine and application thereof
InactiveCN106139139AThe effective amount of antigen is stableLess side effectsViral antigen ingredientsVirus peptidesImmune effectsAdjuvant
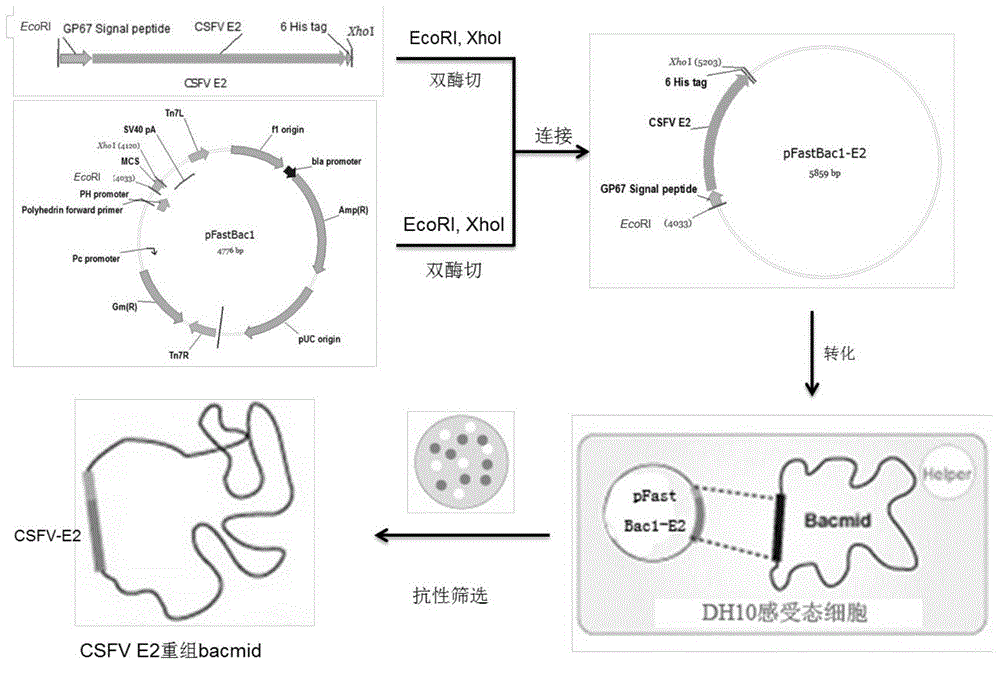
The invention provides a classical swine fever E2 subunit vaccine and a preparation method thereof. The preparation method specifically comprises the steps that a Baculovirus Carrier Expression System is used for expressing a large amount of recombination classical swing fever E2 protein in insect cells, and the classical swine fever E2 subunit vaccine with a good immune effect is developed. The Bac-to-Bac Baculovirus Carrier Expression System is used for expressing the classical swine fever E2 protein. The classical swine fever E2 protein and an adjuvant 201R are emulsified into the novel subunit vaccine, and by means of piglet immune challenge tests, it is verified that the subunit vaccine has a good immune protection effect.
Owner:北京大北农科技集团股份有限公司动物医学研究中心 +3
Coating composition
ActiveCN101591483AImprove corrosion resistanceImprove the bactericidal effectAlkali metal silicate coatingsAnti-corrosive paintsTO-18Emulsion
The invention discloses a coating composition, which comprises the following components in percentage by weight: 36 to 48 percent of water, 0.1 to 0.5 percent of hydrophobic assistant, 0.1 to 0.3 percent of wetting agent, 9 to 18 percent of titanium dioxide, 20 to 35 percent of potassium silicate solution and 5 to 15 percent of elastic emulsion. In the coating composition, potassium silicate solution is adopted as a film forming agent, and the stable and high pH value of the potassium silicate solution provides the coating composition with excellent sterilization effect to avoid the use of antiseptics, reduce harmful gases given out by the coating into the air and make the coating composition more environmentally-friendly. Saul challenge tests prove that the coating prepared by the technical proposal of the invention has high corrosion-resistance.
Owner:CHINA PAINT MFG CO SHENZHEN
Efficacy test method of infectious bronchitis vaccines and application thereof
InactiveCN101957362AReduced measurement timeReduce operating errorsBiological testingInfectious bronchitisQuality control
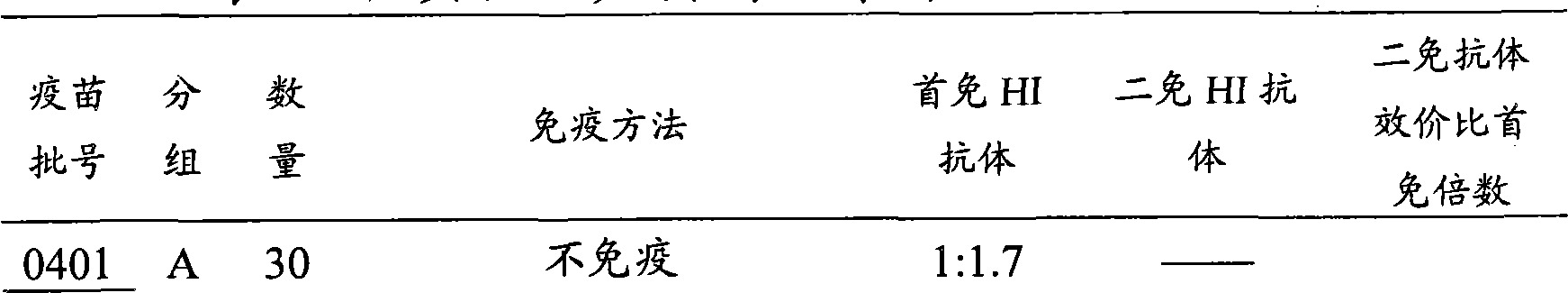
The invention discloses an efficacy test method of infectious bronchitis inactivated vaccines, which can be applied to vaccine quality control specifically comprising challenge protection rate control and egg-laying efficacy control. Experiments prove that the invention uses the method for detecting the HI antibody titer to replace the animal challenge test, and the method has the characteristics of simpleness, convenience, rapidness, accurate results, strong repeatability, strong specificity and the like and has the significance of popularization.
Owner:PU LIKE BIO ENG
Method for producing type 2 capsid protein grain vaccine of porcine circovirus by pichia pastoris expression system
InactiveCN103169961AImprove solubilityHigh expressionViral antigen ingredientsVirus peptidesEscherichia coliPichia pastoris
The invention provides a method for producing type 2 capsid protein grain vaccine of porcine circovirus by a pichia pastoris expression system. The method comprises the following steps of: based on a PCV2-TJ strain gene sequence in GenBank as a template, obtaining a mature coding sequence of PCV-2 capsid protein Cap by amplification by a normal PCR method; connecting the gene with a capsid protein secreting type expression carrier pP-secSUMO3 to construct a pP-secSUMO3-Cap recombinant plasmid and amplifying by a heat stock method to convert escherichia coli JMS115; purifying the positive recombinant plasmid and electrically converting pichia pastoris strain GS115 by an electric converter of BioRad company; sieving high copy strains according to concentration gradient of antibiotic ZeocinTm; and respectively extracting genomic DNAs of the high copy strains as the template to perform PCR reaction to identify positive clone. The piglet challenge test verifies that the grain vaccine has good immunoprotection effect.
Owner:BEIJING DAWEIJIA BIOTECH SHARE CO LTD
Compositions, uses, and method of making wound care products from naturally occurring food ingredients
InactiveUS20090036413A1Good effectReduce excessSalicyclic acid active ingredientsBiocideFood additiveGuideline
Rationally designed wound care products made entirely of naturally occurring food ingredients that can be standardized and made available for the mass market using good manufacturing practice (GMP) guidelines, optionally, a safe food additive can be added. These products: are safe and effective; have an osmotic pressure compatible with optimal healing; are buffered to maintain optimal pH throughout the healing process; provide a protective barrier from further irritation and insult; control bacteria, viruses and fungi found in the skin and mucosa; nourish wounds; control excessive prolonged inflammation and thereby minimize scarring; minimize allergenic and irritation potential; are easy to use or apply; pass the preservative challenge test required for products intended for multiple use; contain fragrant essential oils to take advantage of the benefits provided by aromatherapy; can be individually optimized based on the diet of an individual or group of people.
Owner:BILL MCANALLEY & ASSOCS
Indometacin and albuterol suppository, preparation method, detection method and application thereof
ActiveCN102670593ASolve solubilitySolving Dispersion ProblemsOrganic active ingredientsComponent separationIndometacinSalbutamol
The invention provides an indometacin and albuterol suppository and a preparation method of the suppository; the suppository comprises effective dose of indometacin, effective dose of albuterol, a dispersing agent, emulsifier and a molding agent. The preparation method comprises the steps of dissolving the indometacin and the albuterol in the dispersing agent, then mixing with the molten molding agent and the emulsifier, and homogenizing to obtain the suppository. The suppository provided by the invention is featured with smooth production and uniform quality; and auxiliary materials do not influence content detection; wherein content detection recovery rate of the albuterol reaches 97%, and content uniformity A+1.8S is less than 10, and is superior to a requirement that content uniformity A+1.8S of a suppository is less than 25 (Department 2 of Chinese Pharmacopoeia 2005 edition, Page 75 of Appendix XE); and layering and precipitation do not appear after the suppository is subjected to a challenge test at 45 degrees centigrade for 72 hr.
Owner:LIVZON GROUP LIVZON PHARMA FACTORY +1
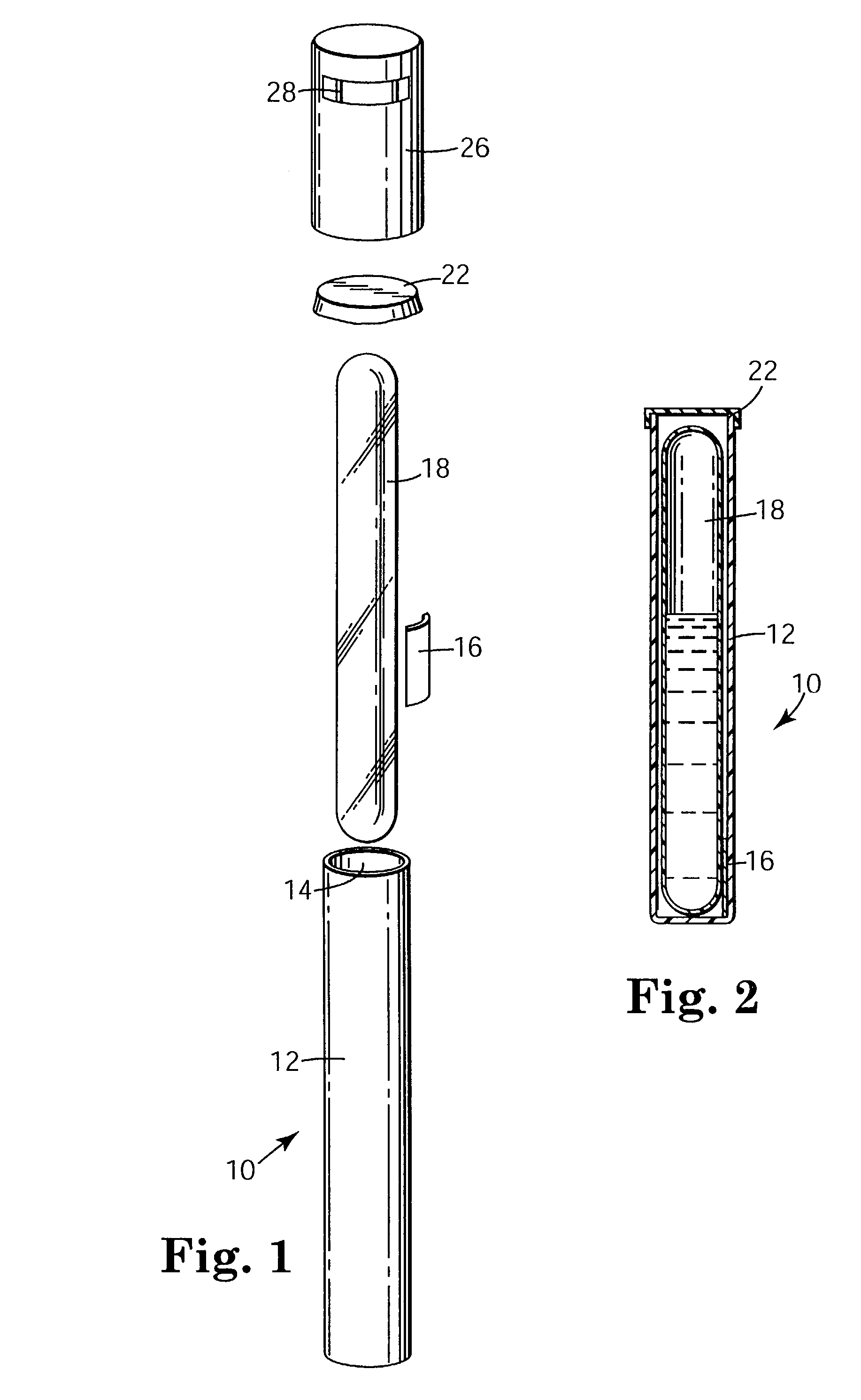
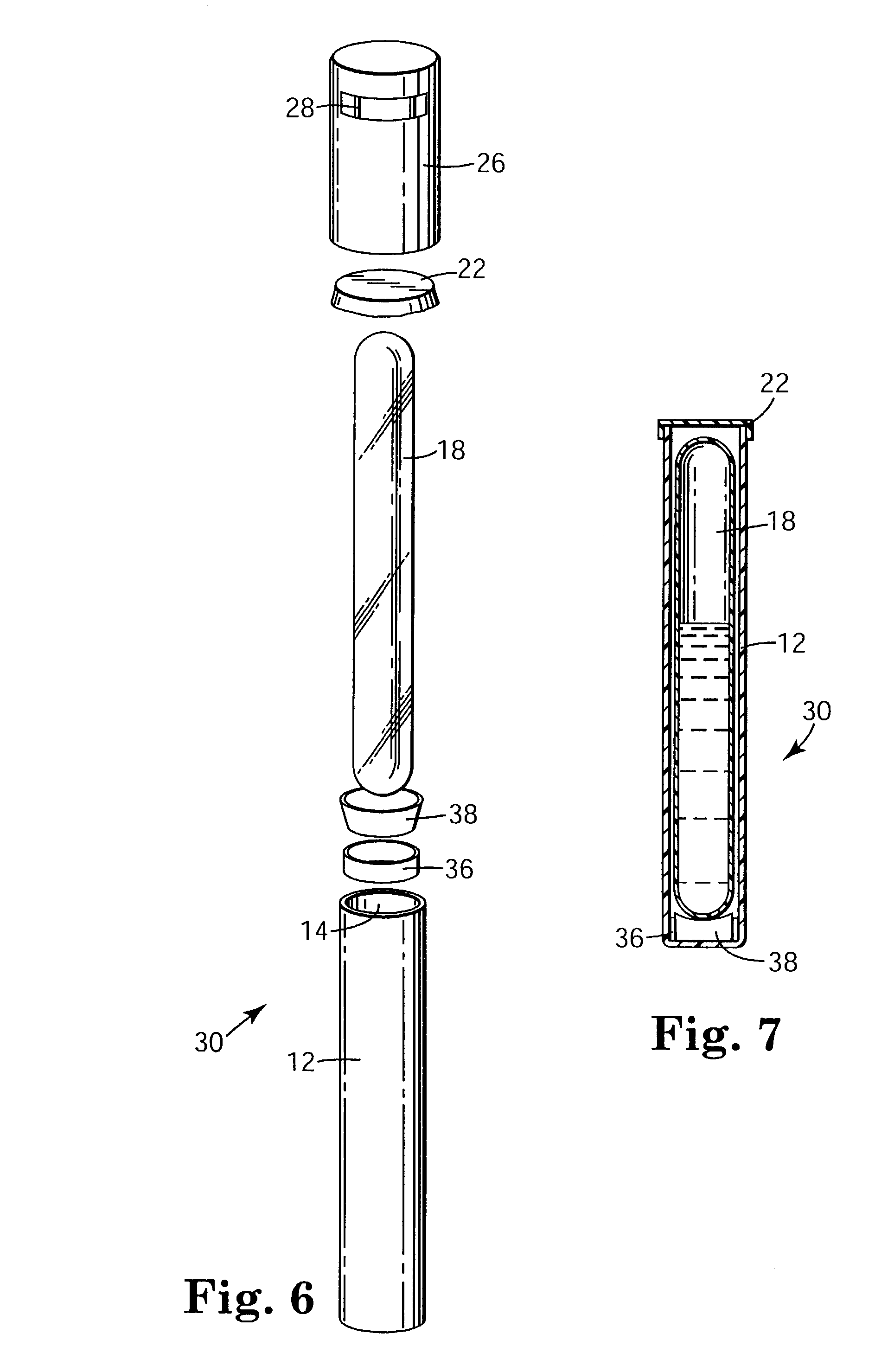
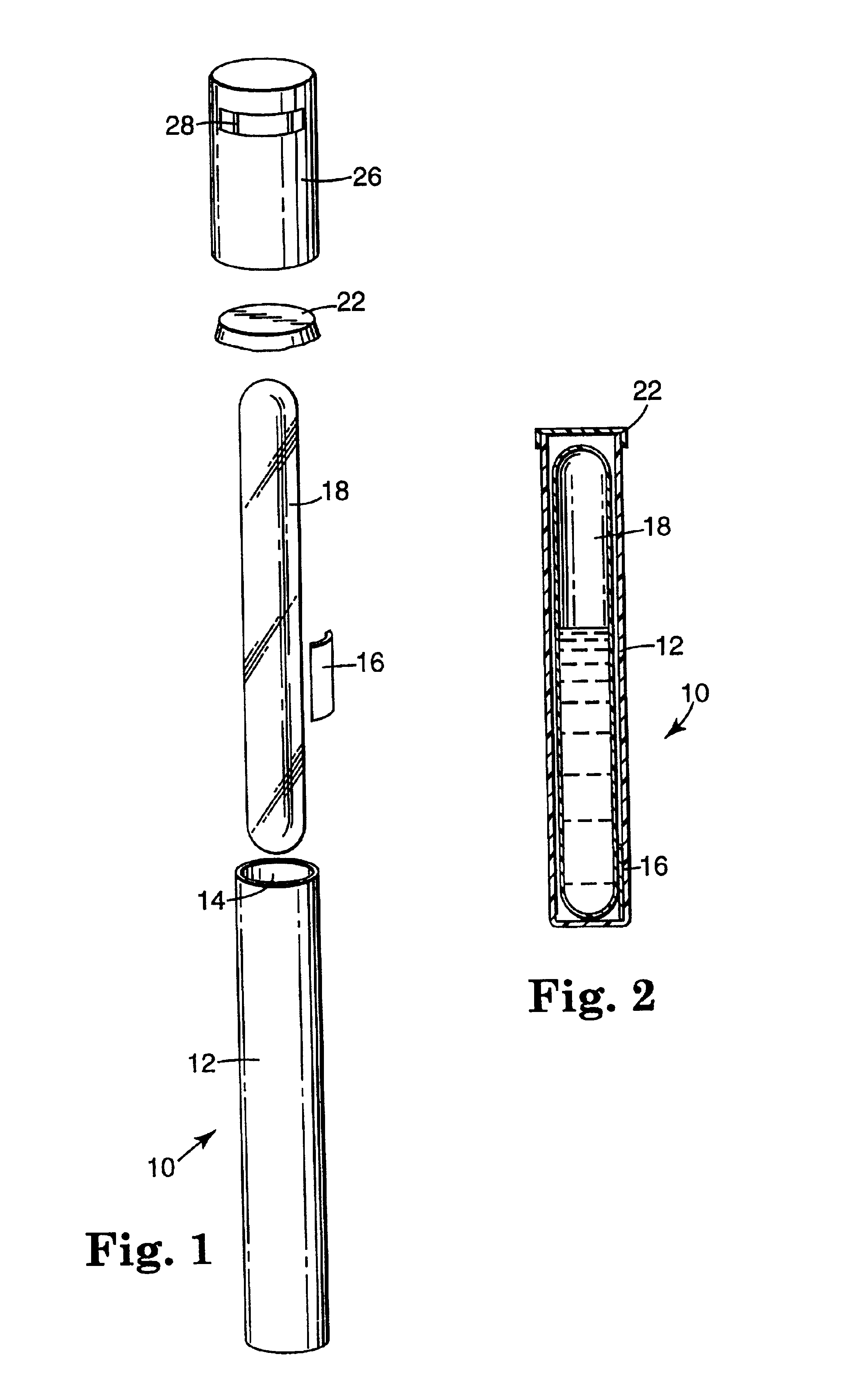
Sterilization process challenge test tube
The invention provides a sterilization process challenge test tube. The sterilization process challenge test tube comprises a tube body, a steam damping structure, an optional porous material and a sterilization indicator, wherein one end of the tube body is provided with an opening and the tube body is composed of a transparent chamber for placing the sterilization indicator, a chamber for placing the optional porous material and a chamber for placing the steam damping structure; and in the sterilization process, steam sequentially passes through the opening and the steam damping structure and finally arrives at the sterilization indicator, wherein the optional porous material can be placed between the steam damping structure and the opening or the steam damping structure and the sterilization indicator.
Owner:明尼苏达矿业制造医用器材(上海)有限公司
Preparation method and application of Brucella multi-epitope fusion protein vaccine
The invention discloses a Brucella multi-epitope fusion protein antigen. Amino acid sequences of dominant epitopes of Brucella outer membrane proteins BP26, OMP31, OMP16 and OMP2b are in series connection to construct a fusion protein gene to express the proteins, and a Brucella multi-epitope fusion protein antigen vaccine is prepared. Mice challenge tests show that the Brucella multi-epitope fusion protein antigen vaccine plays a role in immune protection in Brucella infection.
Owner:JILIN UNIV
PCV2d (porcine circovirus type 2) virus-like particle vaccine and preparation method thereof
InactiveCN108355131ADoes not affect formDoes not affect sizeViral antigen ingredientsVirus peptidesEscherichia coliPhylogenetic tree
The invention relates to a PCV2d (porcine circovirus type 2) virus-like particle vaccine and a preparation method thereof. By means of epidemiological analysis for PCV2d strains of China, comparison of amino acid sequence information of a large quantity of collected strains as well as phylogenetic tree analysis, a Cap gene of a currently epidemic PCV2d strain in China is selected, PCV2d Cap protein is effectively expressed by an Escherichia coli prokaryotic expression system through codon sequence optimization, PCV2d virus-like particles are prepared successfully by purification and assembly in an in-vitro assembly and dialysis buffer solution, and form, size and concentration of the virus-like particles are not affected when the virus-like particles are placed for 6 months in a storage buffer solution at 4 DEG C and subzero 20 DEG C; the prepared PCV2d virus-like particle vaccine immunizes 21-day-old piglets, and a PCV2d challenge test proves that the vaccine has a good protecting effect for the piglets.
Owner:JIANGSU NANNONG HI TECH +1
Application of platycarya strobilacea infructescence extract
ActiveCN104814272AHas a growth-promoting effectHas antibacterial propertiesAntibacterial agentsMetabolism disorderDiseaseGrowth promoting
An application of a platycarya strobilacea infructescence extract. In particular, the invention provides the application of the platycarya strobilacea infructescence extract in preparation of an additive for increasing the yield of aquatic products and preventing and treating diseases of aquatic products, wherein the aquatic products includes fish or shrimp, the fish is epinepheius awoara. The platycarya strobilacea infructescence extract is a water extract of platycarya strobilacea infructescence. In the invention, the extract is prepared with water as an extraction solvent so that an extraction process is simple, economic, green, environment-friendly and safe. With the plant extract feed additive for breeding the epinepheius awoara, a growth promoting effect is achieved. A toxicity challenge test proves that the feed additive has an antibacterial capability on vibrio parahaemolyticus.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
Calcium challenge test for detecting calcium homeostasis disorders
The invention provides simple methods for detecting disorders of a subject's calcium homeostasis. The methods include administering a calcium salt to the subject and observing the effect of this dose of calcium on calcium levels in the subject's bodily fluids and / or tissues. The methods are useful to detect certain calcium homeostasis disorders or a predisposition for such disorders including adynamic bone disease and soft tissue calcification disorders, which are difficult to detect by other methods.
Owner:SCANTIBODIES LAB
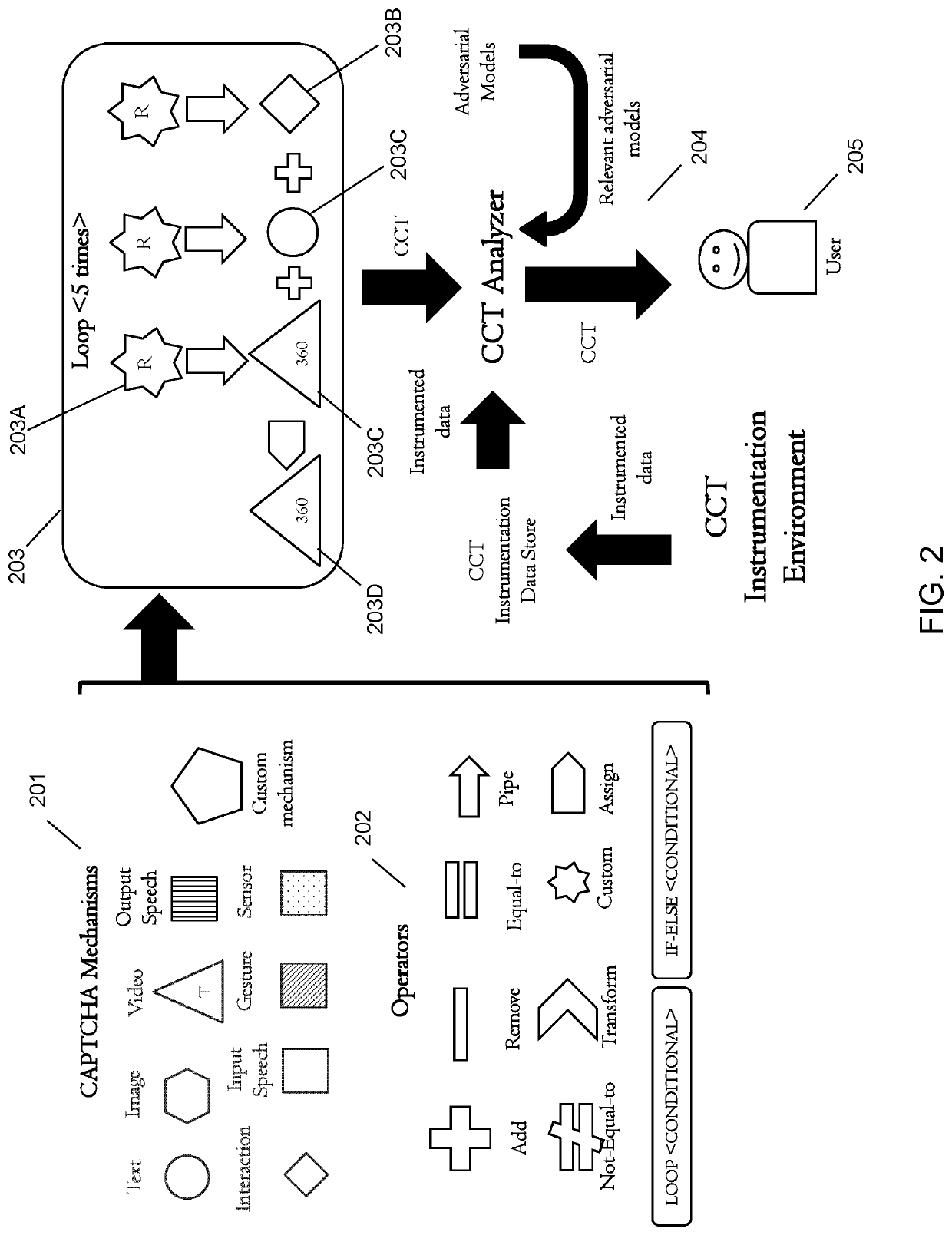
Composite challenge task generation and deployment
One embodiment provides a method, including: receiving at least two challenge test mechanisms of different challenge test modalities, wherein a challenge test mechanism comprises a challenge portion of a challenge-response test for distinguishing between a human operator and a computer; receiving challenge test operators for combining the at least two challenge test mechanisms; generating a composite challenge task by combining the at least two challenge test mechanisms using the identified challenge test operators; identifying any errors in the composite challenge task by running the composite challenge task; evaluating the composite challenge task to determine (i) a challenge difficulty for a human operator and (ii) a challenge difficulty for a computer; and implementing the composite challenge task if (i) no errors are identified at the composite challenge task analyzer, (ii) the challenge difficulty for a human operator is below a predetermined threshold, and (iii) the challenge difficulty for a computer is above a predetermined threshold.
Owner:SWIPEADS HLDG PTY LTD
Method for detection effectiveness of aftosa inactivated vaccines
The invention relates to a method for detecting the effectiveness of aftosa inactivated vaccines. The method comprises the following steps that firstly, each dose of each batch of vaccines immunizes 4 to 8 Balb / C series rats, the immunizing dose of the vaccines can be classified into 1 rat / part, one-second rat / part and one-fourth rat / part, blood sampling is performed on the twenty-eighth day, and serum is separated; secondly, serum specific antibodies are detected through an aftosa inhibiting ELISA method, and if antibody inhibiting ELISA titer-log10X is higher than 2.0 and variable coefficient is lower than 12 percent, the antibodies are judged to be qualified. The invention provides a novel method for detecting the effectiveness of aftosa inactivated vaccine, immunizing Balb / C series rats and detecting serum through the method can properly reflect the effective antigen content in the vaccines, and the method replaces the original animal immunization challenge test.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
In-tank antiseptic antimildew agent composition integrated with dry film for paint
ActiveCN102702839BReduce dosageLow toxicityBiocidePaints with biocidesWater basedMicrobial challenge
The invention relates to an in-tank antiseptic antimildew agent composition integrated with a dry film for paint. The composition is prepared from a water-based paint in-tank antiseptic bactericide, a dry film antimildew agent, a solvent, an aid, a stabilizing agent and water. The in-tank antiseptic antimildew agent composition integrated with a dry film for paint has the advantages of reasonable formula, advanced process, good corrosion and mildew prevention effects, good stability, and the like. When the in-tank antiseptic antimildew agent composition integrated with a dry film for paint is used for paint, salt cluster phenomena can be avoided, an in-tank antiseptic effect can pass a microorganism challenge test, and the dry film mildew-growing grade is a zero grade.
Owner:SHAANXI RES DESIGN INST OF PETROLEUM CHEM IND
Stenotrophomonas maltophilia outer membrane protein and application thereof
InactiveCN106749561AImproving immunogenicityReduce bacterial load in bloodAntibacterial agentsBacterial antigen ingredientsIn vivoVaccine Immunogenicity
The invention discloses a Stenotrophomonas maltophilia outer membrane protein and application thereof. The Smlt4123 protein provided by the invention is any one of the following (a1)-(a3): (a1) protein composed of amino acid sequence disclosed as Sequence 4 in the sequence table; (a2) proteins composed of amino acid sequence of Sequence 4 in the sequence table in the 53rd-240th site from the N terminal; and (a3) Sequence-4-derived protein with the same function as the amino acid sequence disclosed as Sequence 4 subjected to substitution and / or deletion and / or addition of one or more amino acid residues. The Smlt4123 protein has higher immunogenicity. The killing test on the whole blood prepared from the immune serum detects that the protein can lower the bacterium carrying capacity in blood in vitro. The challenge test indicates that the antibody generated by the protein immunization can also lower the bacterium carrying capacity in blood in vivo, has certain protection actions and can be used as a key vaccine candidate molecule.
Owner:中国人民解放军第307医院
Method for producing aviadenovirus 4 type vaccine by using LMH cell line, and vaccine
ActiveCN108969760AGuaranteed potencyImprove immunitySsRNA viruses negative-senseViral antigen ingredientsVaccine antigenAvian adenovirus
The invention belongs to the technical field of veterinary biological products, and particularly relates to a method for producing an aviadenovirus 4 type vaccine by using an LMH cell line, and the vaccine. The LMH cell cultured by utilizing the method provided by the invention is used for culturing an aviadenovirus 4 type, so that the production cost can be greatly reduced, and meanwhile, the titer of the virus is ensured; through the process, within the shortest time 60h, the aviadenovirus 4 type virus with the titer being more than 108.5TCID50 / 0.1ml can be stably obtained, so that comparedwith the prior art, the remarkable technological advantage is realized. According to the vaccine prepared from a vaccine antigen with high titer, the immunity of the vaccine can be remarkably improved, and the immune challenge test result proves that 100 percent of protection can be realized on the attack of the aviadenovirus 4 type.
Owner:广州渔跃生物技术有限公司 +1
Detection method for liquid bacteria intercepting ability of air filtering device/ membrane for infusion
ActiveCN109126475AAdd filter stepPromote growthSemi-permeable membranesMicrobiological testing/measurementSocial benefitsAir filter
The invention aims to provide a detection method for a liquid bacteria intercepting ability of an infusion blood transfusion apparatus. Firstly equipment is installed, and then the following steps arecarried out that an air filtering device / membrane is wet, and wetting liquid residues are verified; a challenging bacterial liquid is prepared, a bacterial liquid state is confirmed, and the volumeof the challenging bacterial liquid is calculated; test pressure values under different challenges are determined; different challenge tests are performed, and results are determined; the method is novel and unique, the operation and use are easy, the method is used for testing the liquid bacteria intercepting ability of the 0.22-micrometer air filtering device / membrane, the use safety of the infusion blood transfusion apparatus is ensured, and the economic and social benefits are significant.
Owner:河南省医疗器械检验所
Preparation method for inactivated vaccine against avian pasteurellosis
InactiveCN105288607AGood immune protectionAntibacterial agentsBacteriaPasteurella aviumPasteurella multocida type
The invention discloses a preparation method for an inactivated vaccine against avian pasteurellosis. The preparation method comprises the following steps: preparation of a strain used for production; preparation of bacterial liquid used for vaccine preparation; inactivation of the bacterial liquid used for vaccine preparation; and preparation of the vaccine. According to the method, avian Pasteurella multocida type A is used as the original strain; the strain undergoes culture in an iron-limited environment so as to obtain the bacterial liquid eventually; and the bacterial liquid is mixed with a white oil adjuvant so as to prepare the inactivated vaccine against avian pasteurellosis. A local chicken is immunized with the vaccine prepared by using the method, then challenge test is carried out, and results show than a protection rate reaches 100%, so it is proved that the vaccine exerts good immunoprotection effect.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Challenge test for diagnosing subtypes of asd
ActiveUS20190134230A1Compounds screening/testingNervous disorderPositive responseAutism spectrum disorder
The present invention is directed to a method for differentiating between ASD phenotype 1, phenotype 2 and other ASD patients, wherein the method comprises,: administering an Nrf2-activator to a subject, and identifying the subject as an ASD phenotype 1 patient if the subject shows a negative response, as a phenotype 2 patient if he shows a positive response and as another ASD patient if he does not show a positive nor a negative response, wherein the subject is a patient previously diagnosed with idiopathic ASD or a subject displaying clinical signs of ASD. Likewise, the present invention is directed to an Nrf2-activator for use in differentiating between autism spectrum disorder (ASD) phenotype 1 patients, phenotype 2 patients and other ASD patients, wherein the Nrf2-activator is administered to a subject, wherein a phenotype 1 patient is identified by a negative response, a phenotype 2 patient is identified by a positive response and other ASD patients are identified by the absence of a positive and a negative wherein the subject is a patient previously diagnosed with idiopathic ASD or a subject displaying clinical signs of ASD.
Owner:STALICLA SA
Natural cosmetic preservative composition and preparation method thereof
ActiveCN108785197AImprove anti-corrosion performanceEasy to prepareCosmetic preparationsToilet preparationsIrritationPreservative
The invention relates to a natural cosmetic preservative composition and a preparation method thereof. The natural cosmetic preservative composition is prepared from the following raw materials according to a proper weight proportional ratio, wherein the raw materials comprise three natural components of lactic acid bacteria fermentation extract, rice bran extract and peony root bark extract; thecomponents of the raw materials reach the synergistic function. The natural cosmetic preservative composition has the advantages that the preparation method of the preservative composition is optimized; the mixing of the components of the raw materials is specifically treated, so that the anticorrosive ability of the preservative composition and the stability in the cosmetics are improved; the finally prepared preservative composition has good cosmetic anticorrosive function; proofed by experiment, the preservative composition can pass through the anticorrosive challenge test, and the safe andnon-irritation effects are realized for skin.
Owner:上海棠美生物科技有限公司
Preparation method and application of staphylococcus aureus TAF fusion protein
ActiveCN105384800AGood immune protectionImproving immunogenicityAntibacterial agentsAntibody mimetics/scaffoldsStaphylococcus cohniiImmunodominance
The invention relates to a staphylococcus aureus FnBPA immunodominance segment and provides a coding gene of the immunodominance segment. The invention also relates to a fusion protein containing the staphylococcus aureus FnBPA immunodominance segment. The fusion protein is prepared by fusing TRAP protein of staphylococcus aureus, alpha-hemolysin immunodominance segment, and staphylococcus aureus FnBPA immunodominance segment. The invention also provides a coding gene, a preparation method and applications of the fusion protein. The TAF fusion protein has been applied to mouse immunity and challenge tests, and the test results show that TAF fusion protein has a good immuno-protective effect, is an ideal vaccine antigen of staphylococcus aureus, and has an important value in the development and application of novel vaccines.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Pure plant preservative composition as well as preparation method thereof and application thereof to facial masks
ActiveCN110075034AGood antibacterial effectImprove the safety of useCosmetic preparationsToilet preparationsPolyolPreservative
The invention relates to a pure plant preservative composition as well as a preparation method thereof and application thereof to facial masks. The pure plant preservative composition is prepared fromthe following raw material components in parts by weight: 5 to 15 parts of polygonum cuspidatum extract, 5 to 15 parts of pepper extract, 5 to 15 parts of chrysanthemum extract, 5 to 15 parts of rosemary extract, 5 to 15 parts of liquorice extract and 2 to 5 parts of tea extract. The pure preservative composition is formed by compounding six pure plant extracts and has significant bacteriostaticeffect on various pathogenic bacteria through synergistic effect of the components; and the pure plant preservative composition consists of the pure plant natural components without adding polyol, canpass corrosion protection challenge test when applied to the facial masks, and has extremely high use safety.
Owner:SHANGHAI YAOJIAN BIO TECH
VP2 gene and NP gene recombinant adenovirus and application thereof
ActiveCN108060141AVirus peptidesMicroorganism based processesDisk diffusion susceptibility testAgar diffusion test
The invention provides a VP2 gene and NP gene recombinant adenovirus and application thereof. The preparation method comprises the following steps: respectively amplifying VP2 genes and NP genes fromSD strains of IBDV (Infectious Bursal Disease Virus) and AIV (Avian Influenza Virus), cloning the obtained target genes into shuttle vectors pDC315-MCS-EGFP, performing co-transfection on a recombinant adenovirus shuttle plasmid pDC315-VP2-NP-EGFP, an adenovirus macroskeleton pBHGlox(delta)E1 and 3Cre in HEK293 cells by utilizing a lipofection transfection method, recombining and packaging, thereby obtaining the recombinant adenovirus pBH-VP2-NP-EGFP containing the VP2 genes and NP genes. The recombinant adenovirus is capable of stimulating the body to produce an antibody, the IBDV antibody content determined by an agar diffusion test reaches an effective antibody level, and the AIV antibody content determined by a hemagglutination inhibition test method is obviously superior to that in acontrol group pBH-EGFP; and the challenge test results show that the protection rate reaches 90%.
Owner:TIANJIN RINGPU BIO TECH
Indometacin and albuterol suppository, preparation method, detection method and application thereof
ActiveCN102670593BEnsure safetyGuaranteed validityOrganic active ingredientsComponent separationIndometacinSalbutamol
Owner:LIVZON GROUP LIVZON PHARMA FACTORY +1
Pre-vacuum pressure steam sterilization fast biology challenge test bag
PendingCN109045334AReduce use costImprove energy savingLavatory sanitoryHeatVacuum pressureMetallurgy
The invention provides a pre-vacuum pressure steam sterilization fast biology challenge test bag. A microporous ceramic container is prepared from reusable microporous ceramic, and the microporous ceramic container comprises a microporous ceramic container upper cover and a microporous ceramic container lower cover, wherein the microporous ceramic container upper cover is arranged over the microporous ceramic container lower cover, and a sealing gasket is arranged between the microporous ceramic container upper cover and the microporous ceramic container lower cover; a fast biology indicator is placed in the microporous ceramic container; the sealing gasket is clamped by the microporous ceramic container upper cover and the microporous ceramic container upper cover through a clamping device, and the clamping device comprises a fastening frame or / and a clamp. According to the pre-vacuum pressure steam sterilization fast biology challenge test bag, the microporous ceramic is used as a reusable auxiliary, and the auxiliary can be used repeatedly, so that not only is the use cost reduced, but also good effects of energy saving, consumption reduction, environmental protection and emission reduction can be achieved.
Owner:衡水诺盾生物科技有限公司
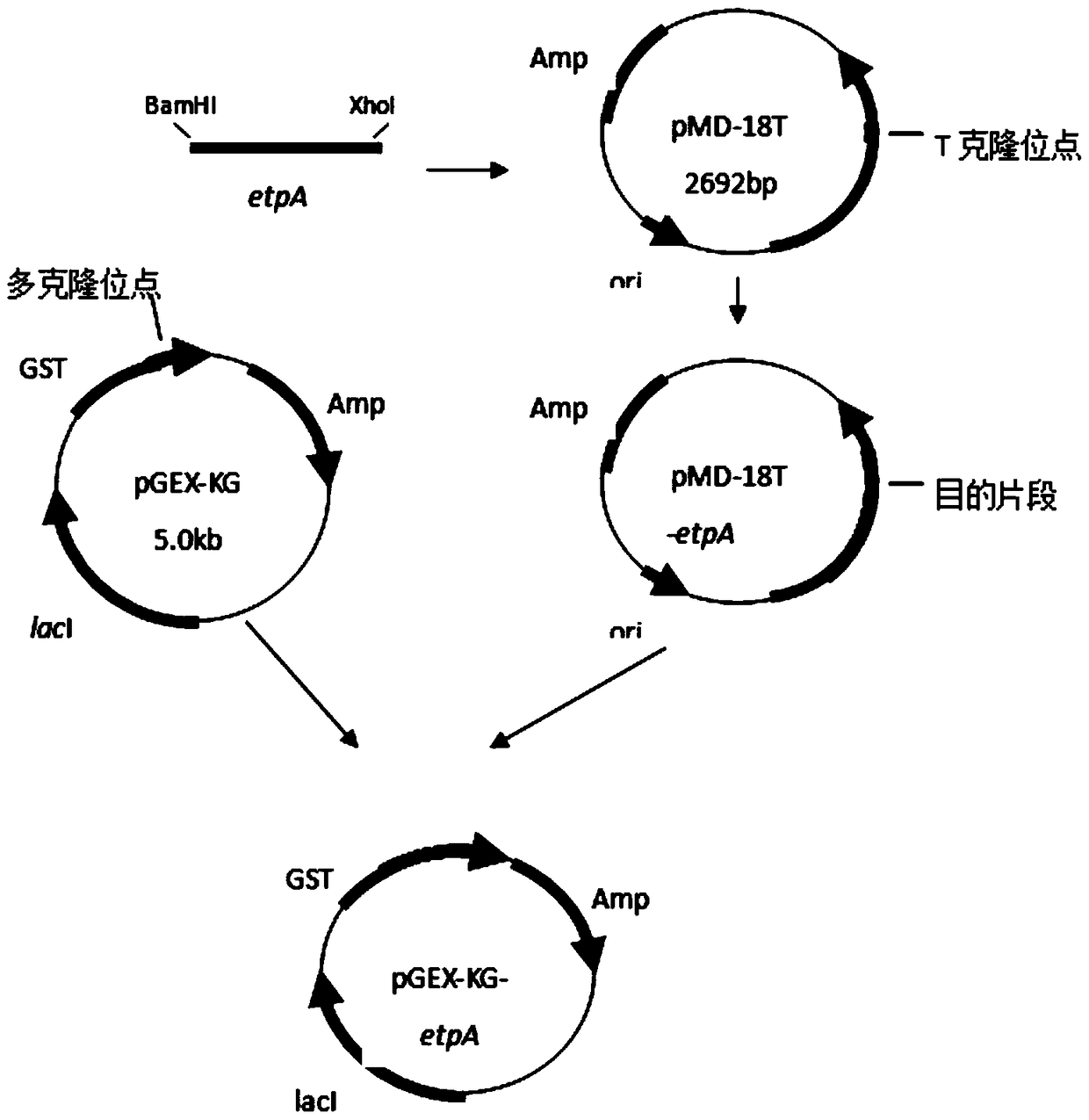
A kind of human source enterotoxigenic Escherichia coli adhesin etpa fusion protein and application thereof
ActiveCN104789584BHigh potencyHigh antibody titerAntibacterial agentsEgg immunoglobulinsYolkFhit gene
The invention discloses a fusion protein of human enterotoxin E. coli adhesin EtpA and its application. The invention provides a recombinant plasmid pGEX‑etpA containing the adhesin gene of human enterotoxin E. coli. The plasmid was transformed into Escherichia coli BL21(DE3), and the recombinant expression strain BL21(DE3) / pGEX‑etpA was induced to express the fusion protein EtpA. The expressed recombinant protein was used to actively immunize first-laying hens, and the yolk antibody against this protein was prepared. The extracted yolk antibody verified that the recombinant protein had good antigenicity, and the mouse intestinal challenge test and in vitro experiments proved that The purified egg yolk antibody can inhibit the adhesion of ETEC strain to epithelial cells in mouse intestine and in vitro.
Owner:HUAZHONG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com