Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
83 results about "Fusion inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical Definition of Fusion inhibitor. Fusion inhibitor: A class of antiretroviral drugs that work on the outside of the host CD4 cell to prevent HIV from fusing with and infecting it. Fusion inhibitors act by binding to an envelope protein and blocking the structural changes necessary for the virus to fuse with the host CD4 cell.
Long Acting Biologically Active Conjugates
InactiveUS20070207952A1High level of drugPoor adhesionBiocidePeptide/protein ingredientsImmunodeficiency virusIn vivo
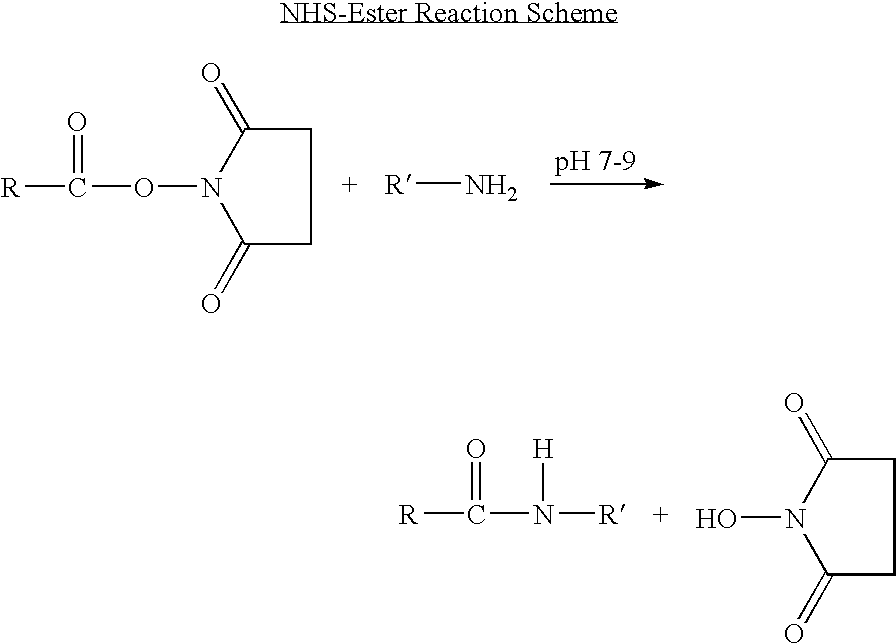
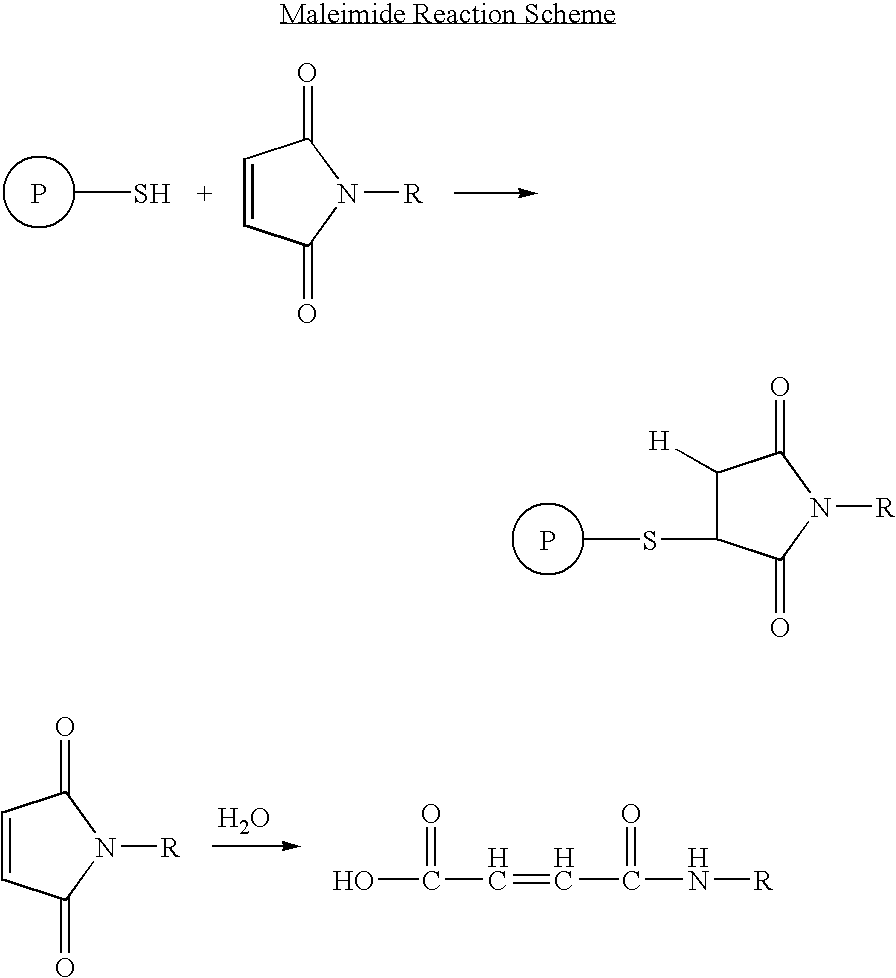
The invention provides biologically active compounds that may be reacted with macromolecules, such as albumin, to form covalent linked complexes wherein the resulting complexes exhibit a desired biological activity in vivo. More specifically, the complexes are isolated complexes comprising a biologically active moiety covalently bound to a linking group and a protein. The complexes are prepared by conjugating a biologically active moiety, for example, a renin inhibitor or a viral fusion inhibitor peptide, with purified and isolated protein. The complexes have extended lifetimes in the bloodstream as compared to the unconjugated molecule, and exhibit biological activity for extended periods of time as compared to the unconjugated molecule. The invention also provides anti-viral compounds that are inhibitors of viral infection and / or exhibit anti-fusiogenic properties. In particular, this invention provides compounds having inhibiting activity against viruses such as human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) and that have extended duration of action for the treatment of viral infections.
Owner:SEQUOIA PHARMACEUTICALS INC
Treatment of HIV and other viral infections using combinatorial therapy
InactiveUS6475491B1Good curative effectLow toxicityBiocidePeptide/protein ingredientsAntiviral drugNon toxicity
Novel antiviral combinations for the treatment or prevention of viral infections, in particular, HIV, are disclosed. This new antiviral therapy employs either DP-178 or DP-107, viral fusion inhibitors, in combination with at least one other antiviral therapeutic agent. The combinations of the invention are better than single therapies alone, and in certain cases are synergistic. The use of DP-178 or DP-107 is an ideal therapy to combine with another antiviral, given both the novel mechanism which this therapeutic blocks HIV transmission and the non-toxicity of the therapeutic.
Owner:TRIMERIS
Dock-and-lock (DNL) constructs for human immunodeficiency virus (HIV) therapy
ActiveUS8481041B2Reduce eliminateReduce and eliminate focusOrganic active ingredientsImmunoglobulins against virusesAntigenAntibody fragments
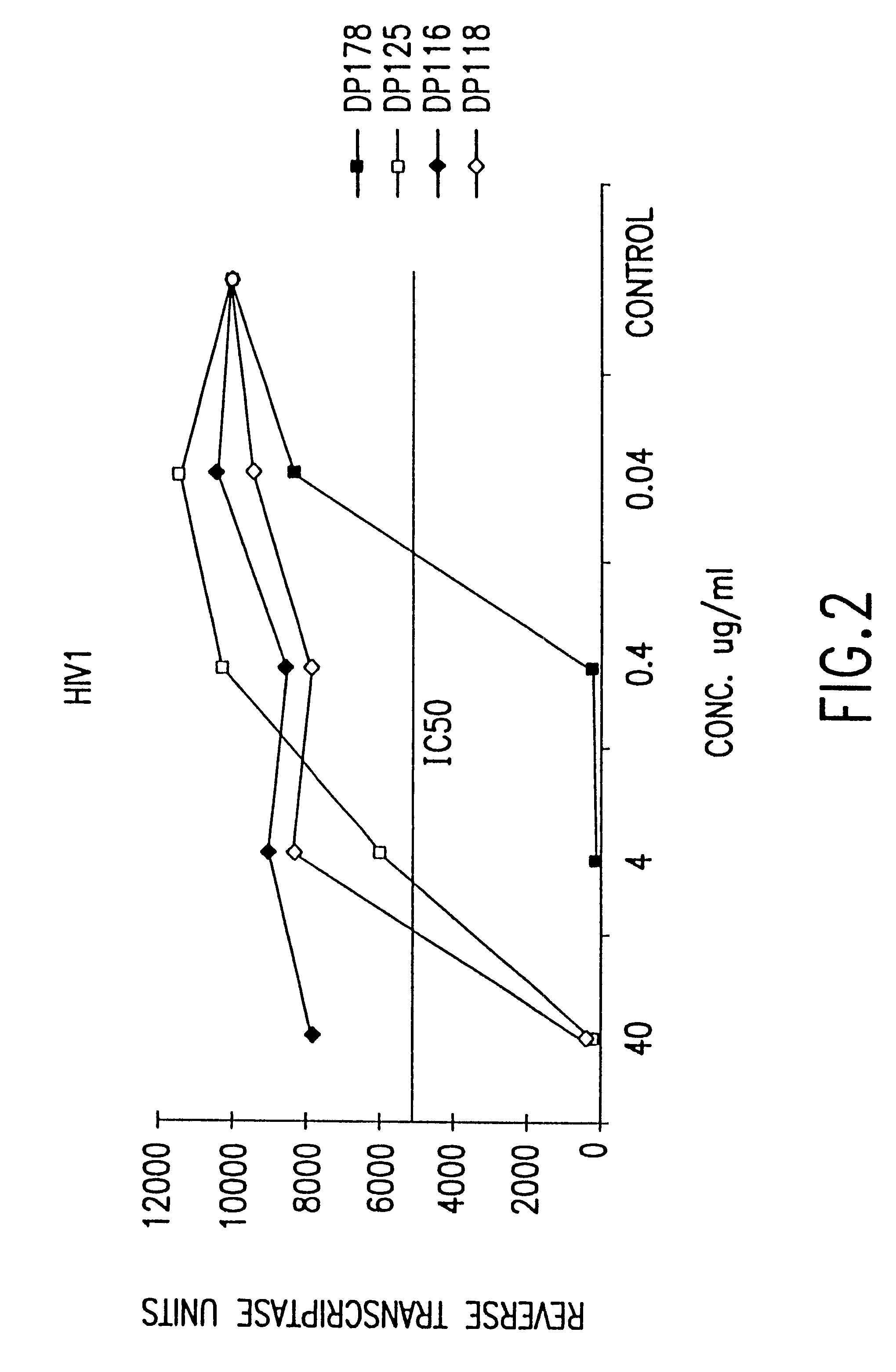
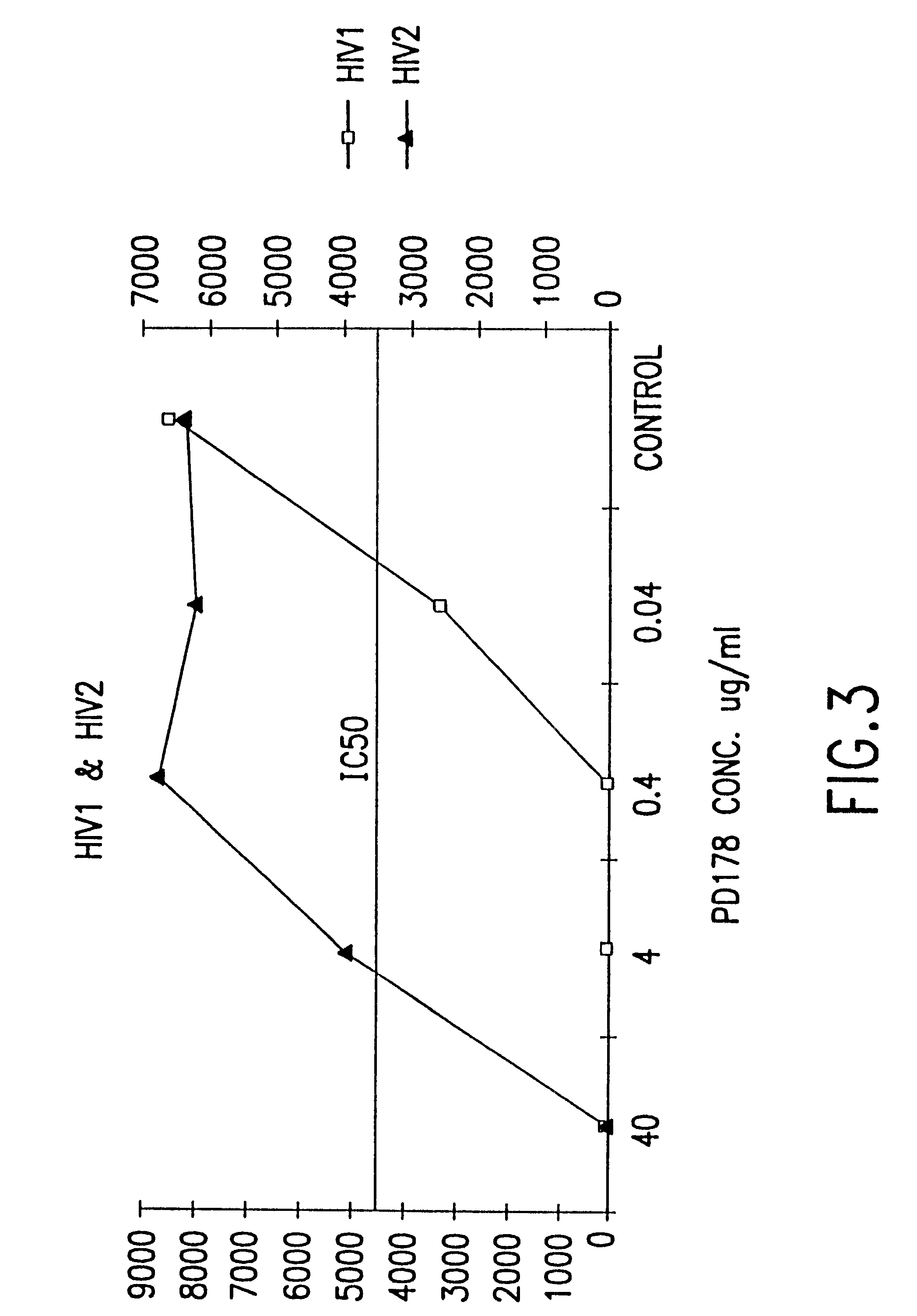
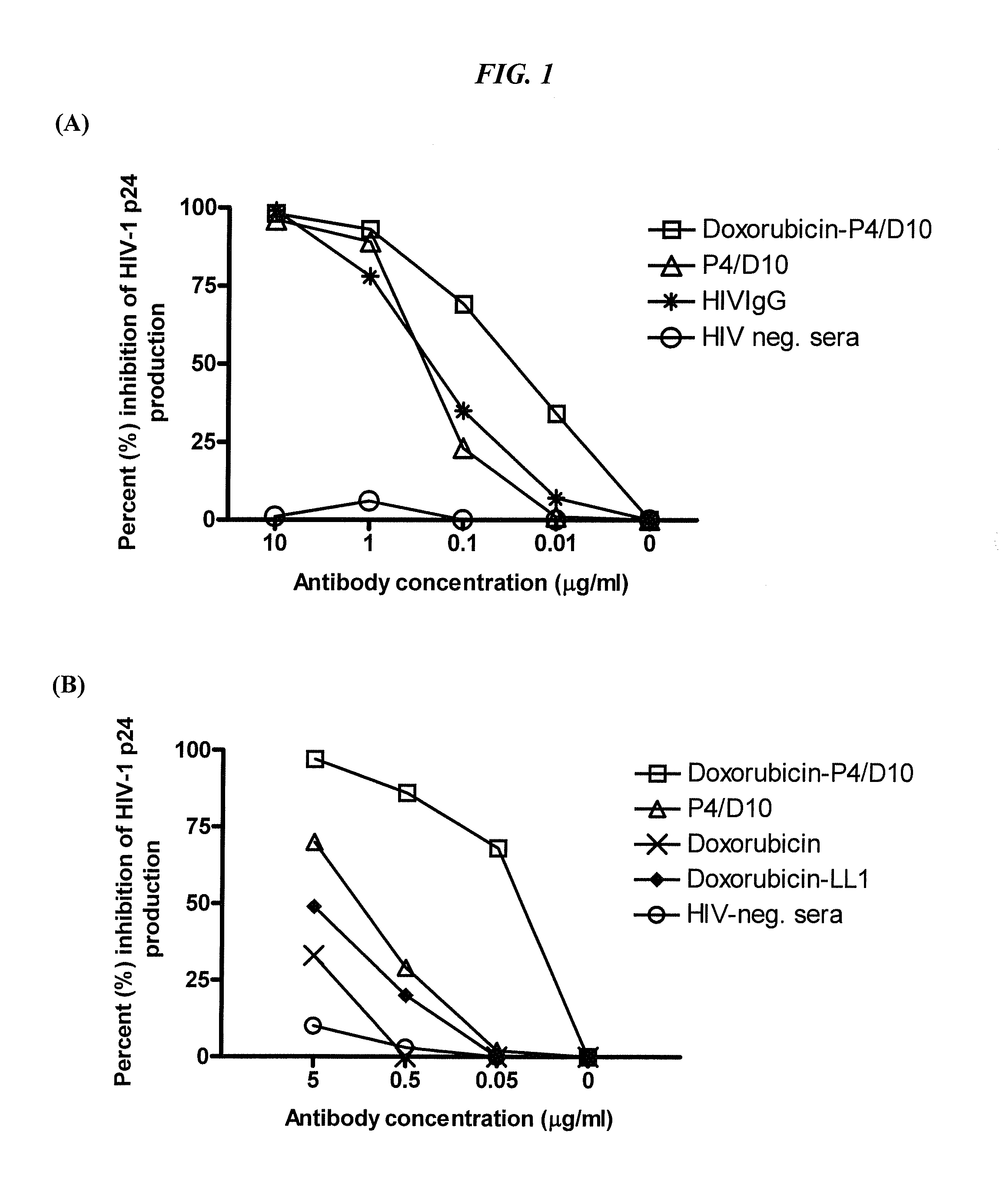
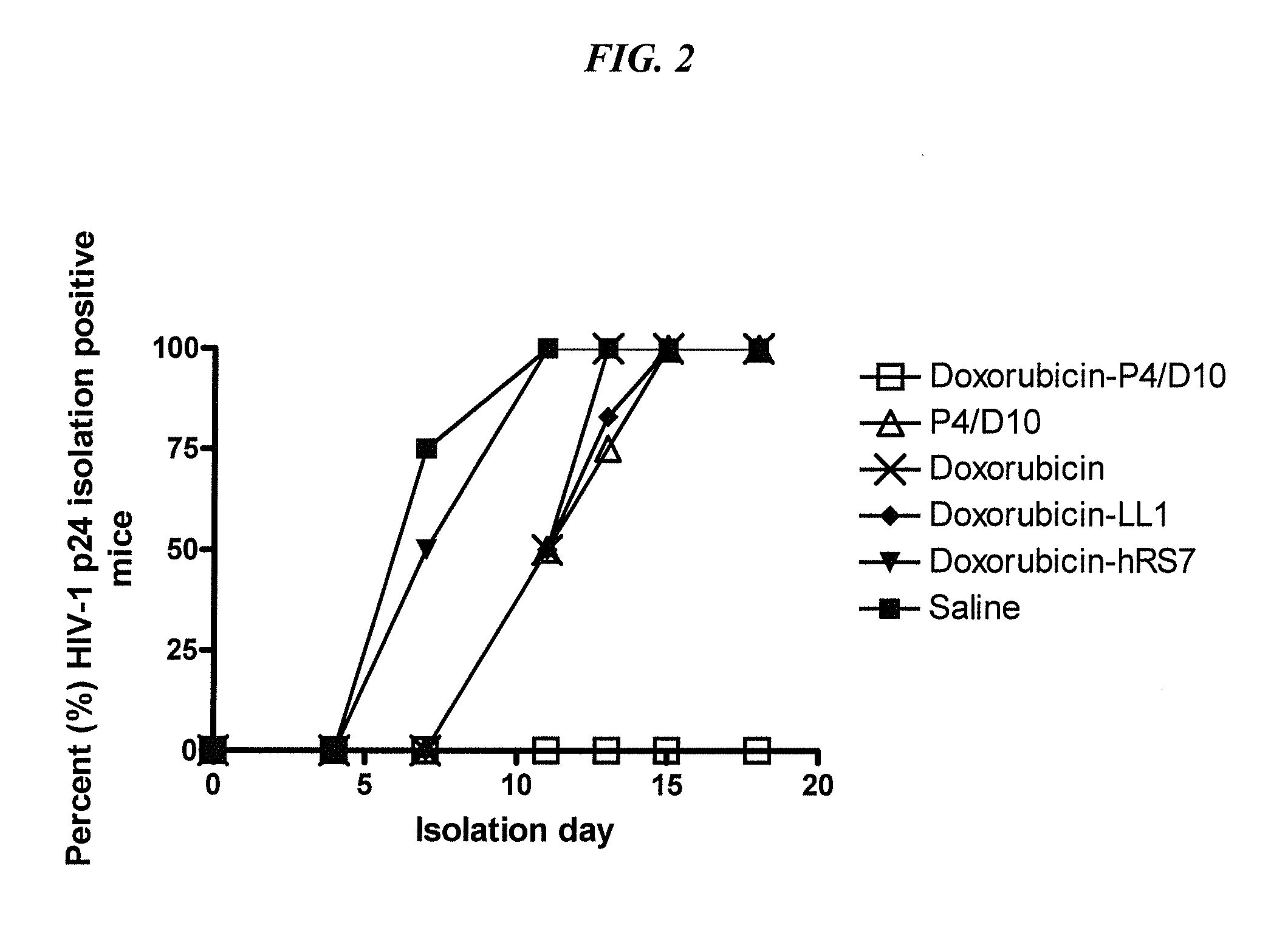
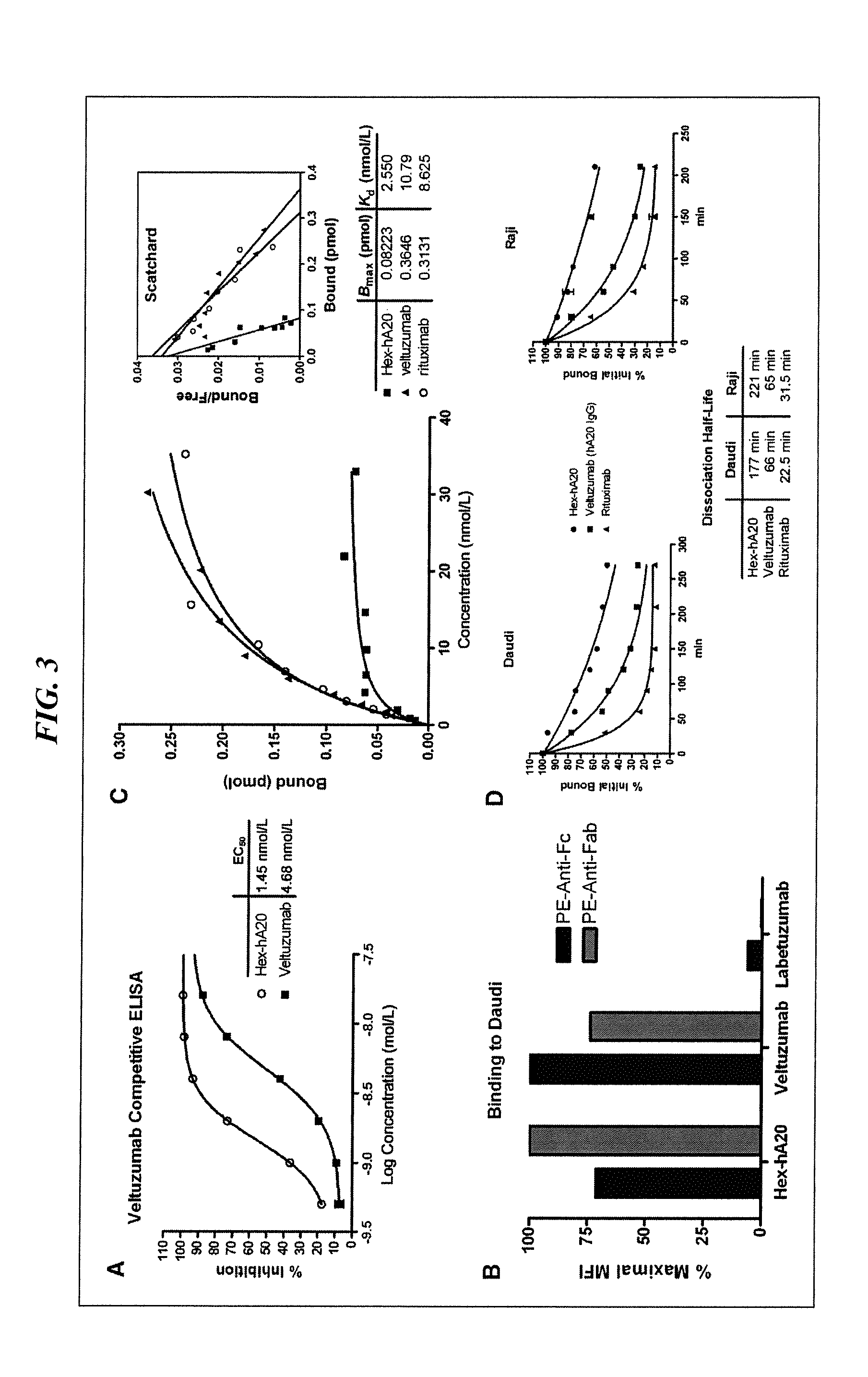
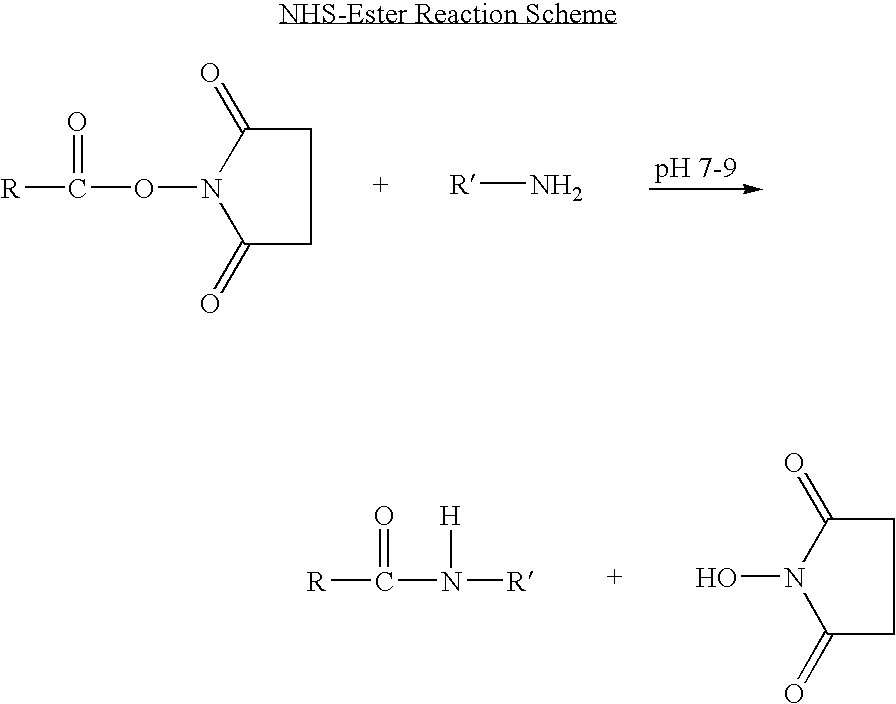
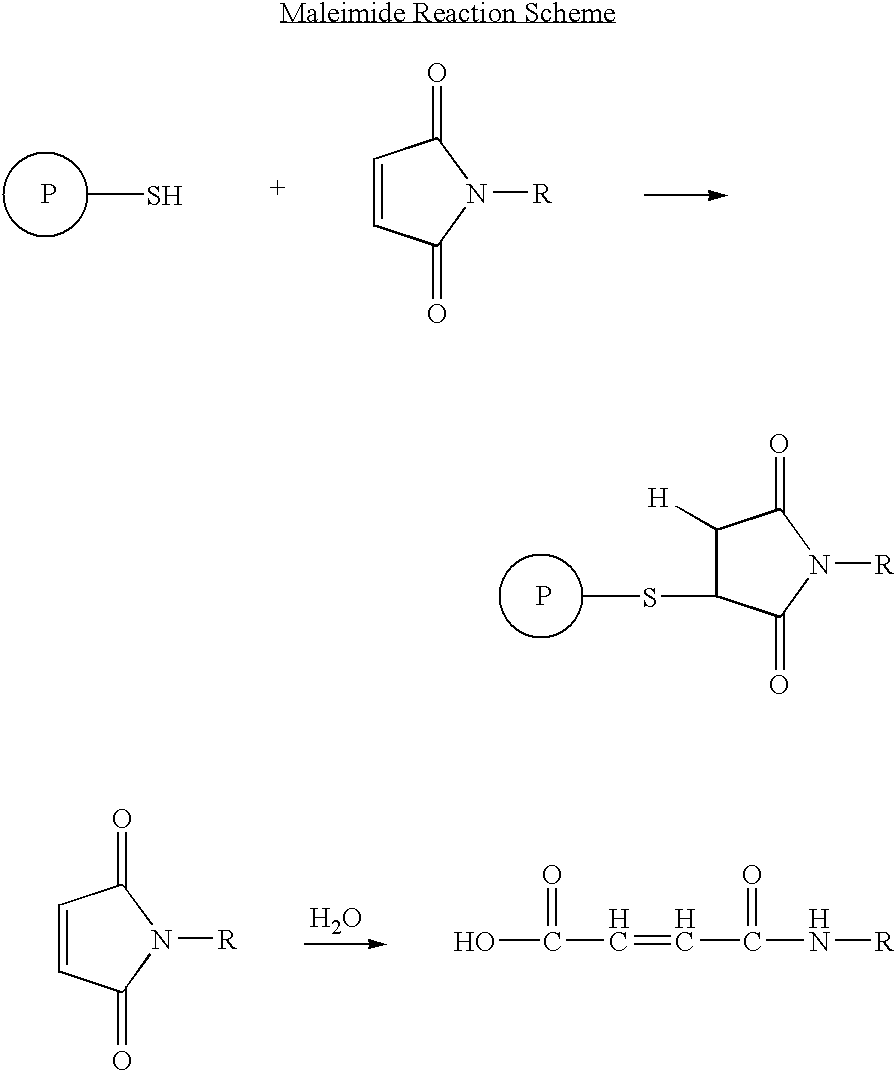
The present invention concerns methods and compositions for treatment of HIV infection in a subject, utilizing a DNL complex comprising at least one anti-HIV therapeutic agent, attached to an antibody, antibody fragment or PEG. In a preferred embodiment, the antibody or fragment binds to an antigen selected from gp120, gp41, CD4 and CCR5. In a more preferred embodiment the antibody is P4 / D10 or 2G12, although other anti-HIV antibodies are known and may be utilized. In a most preferred embodiment, the anti-HIV therapeutic agent is a fusion inhibitor, such as T20, T61, T651, T1249, T2635, CP32M or T-1444, although other anti-HIV therapeutic agents are known and may be utilized. The DNL complex may be administered alone or may be co-administered with one or more additional anti-HIV therapeutic agents.
Owner:IBC PHARMACEUTICALS INC
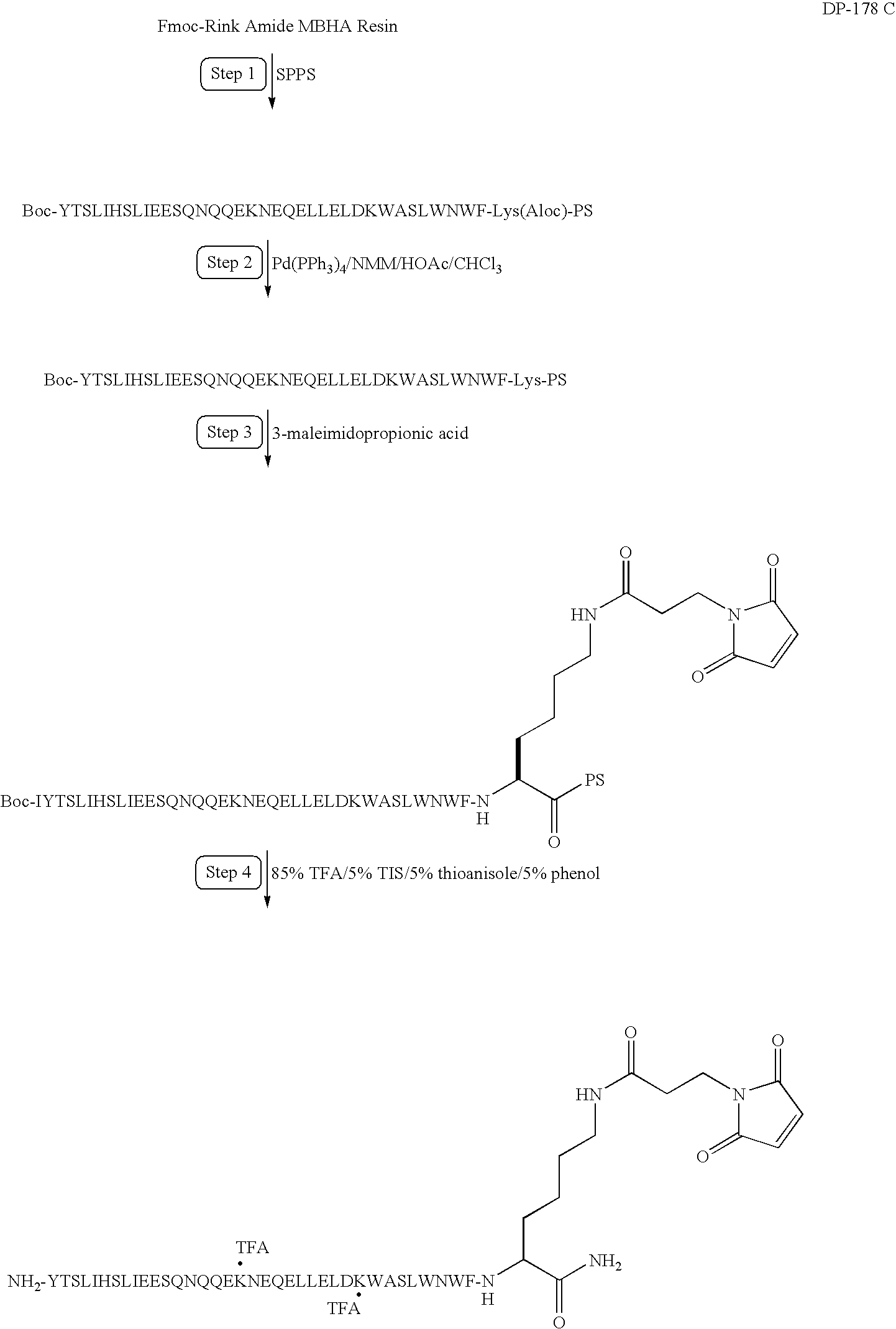
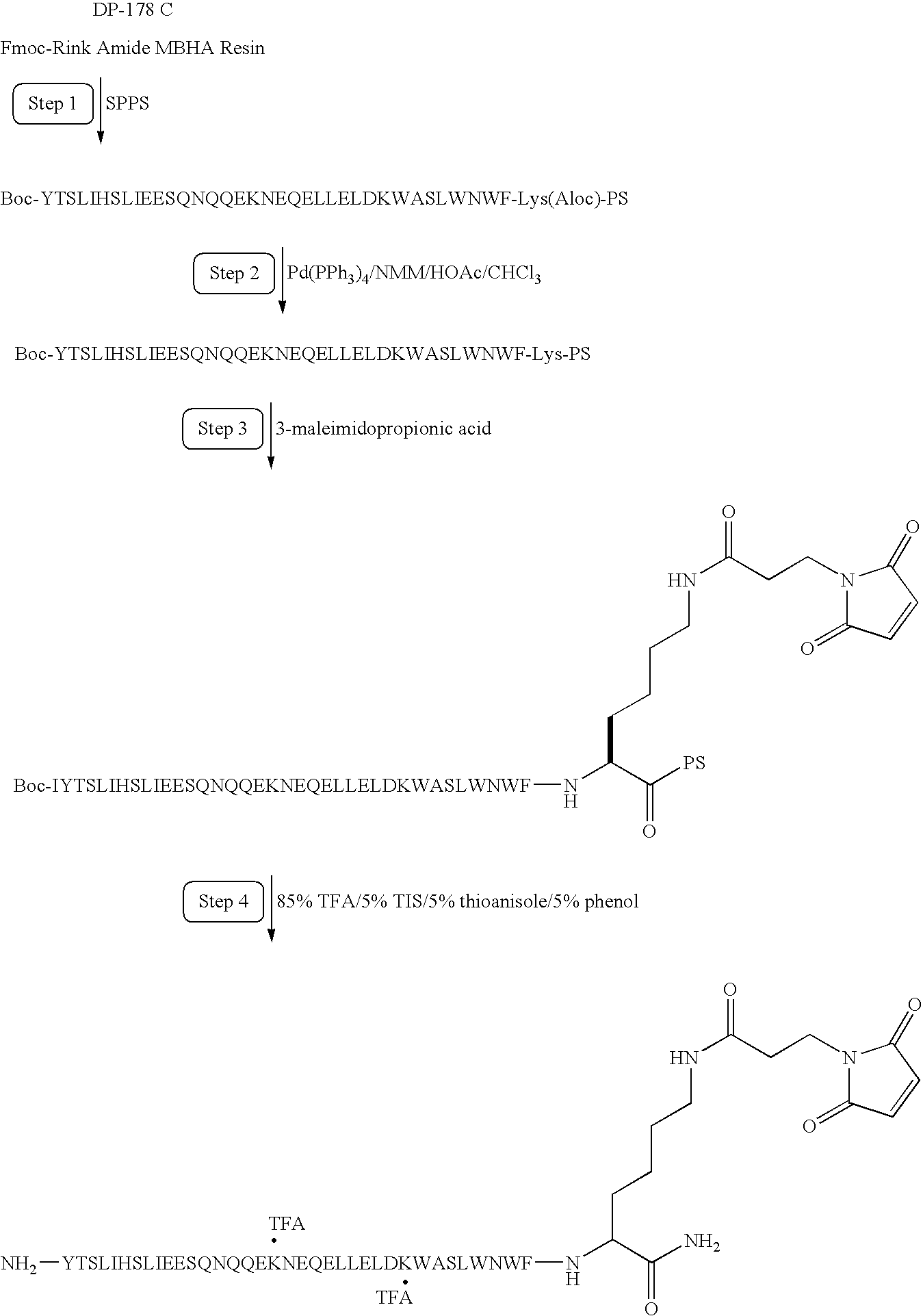
Long-lasting antiviral fusion inhibitor peptide conjugates comprising albumin and human immunodeficiency virus (HIV) peptides
InactiveUS7582301B1Reduce sensitivityImprove in vivo stabilityPeptide/protein ingredientsVirus peptidesHalf-lifeImmunodeficiency virus
Peptides exhibiting anti-viral and anti-fusogenic activity are modified to provide greater stability and improved half-life in vivo. The selected peptides include fusion inhibitors DP178 and DP107 and related peptides and analogs thereof. The modified peptides are capable of forming covalent bonds with one or more blood components, preferably a mobile blood component.
Owner:CONJUCHEM
Long lasting fusion peptide inhibitors of viral infection
InactiveUS7090851B1Improve in vivo stabilityReduce sensitivityVirus peptidesCarrier-bound antigen/hapten ingredientsBlood componentHalf-life
Peptides exhibiting anti-viral and anti-fusogenic activity are modified to provide greater stability and improved half-life in vivo. The selected peptides include fusion inhibitors DP178 and DP107 and related peptides and analogs thereof. The modified peptides are capable of forming covalent bonds with one or more blood components, preferably a mobile blood component.
Owner:CONJUCHEM
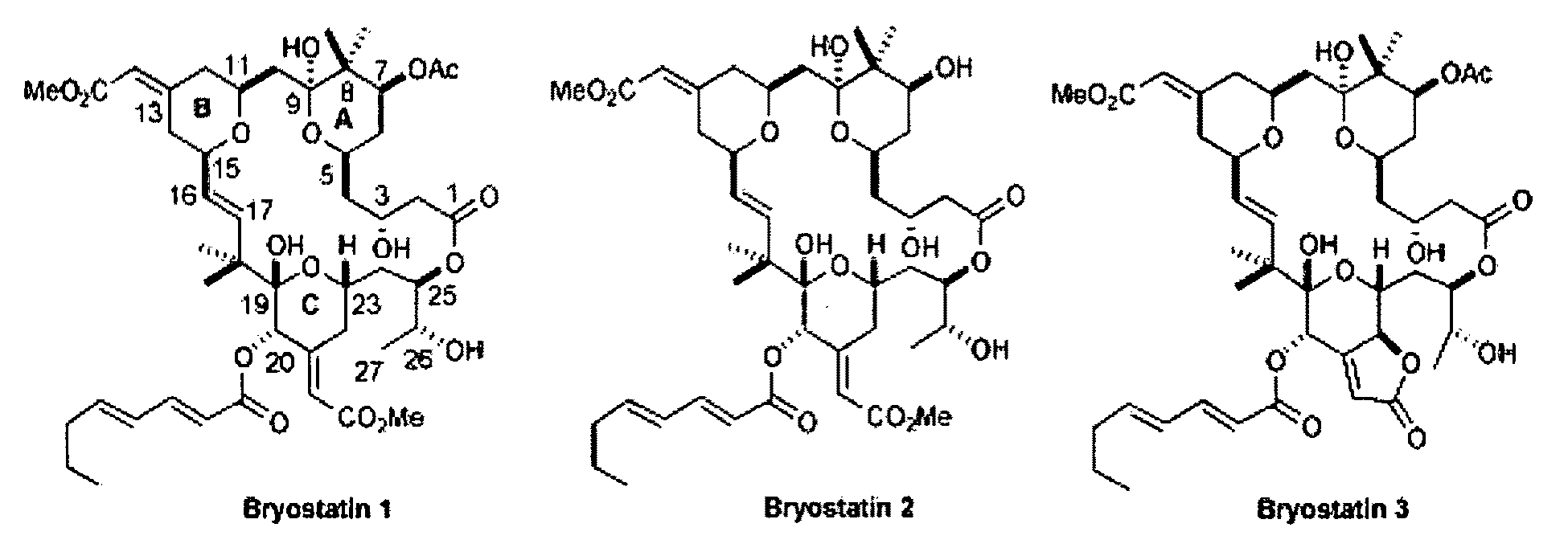
Combination therapy comprising the use of protein kinase C modulators and Histone Deacetylase inhibitors for treating HIV-1 latency
InactiveUS20100166806A1Adverse propertyPrevent HIV-1-induced cytotoxicityBiocideOrganic chemistryReverse transcriptaseHydroxamic acid
The invention relates to a combination of treatments, more particularly a combination treatment for HIV-1 infection. The present invention is directed to the use of bryostatin-1 and their natural and synthetic derivatives for AIDS therapy, in particular to the use of bryostatins in combination with other active drugs such as Histone Deacetylases (HDACs) inhibitors and anti-retrovirals, for the treatment of HIV-1 latency. According to the present invention, we provide a combination therapy for the treatment of HIV-1 latency which employs bryostatin-1 (and analogues) and one of the following HDAC inhibitors; valproic acid, butyrate derivatives, hydroxamic acids and benzamides. While HDACi can be used in continuous dosing protocol, bryostatins can be used following a cyclical dosing protocol. Bryostatins can be formulated in pharmaceutical acceptable carriers including nanoparticles, phospholipids nanosomes and / or biodegradable polymer nanospheres. This combination therapy needs to be used in patients treated with antiretroviral therapy (HIV-1 protease inhibitors, HIV-1 reverse transcriptase inhibitors, HIV-1 integrase inhibitors, CCR5 co-receptor inhibitors and fusion inhibitors).
Owner:APHIOS
Fc chimeric proteins with anti-HIV drugs
InactiveUS20050281829A1Improve stabilityStable HIV chimeric proteinsPeptide/protein ingredientsAntibody mimetics/scaffoldsAntiviral drugImmunoglobulin Fc Fragments
The invention relates to anti viral agents comprised of viral fusion inhibitors and at least a portion of an immunoglobulin constant region. The invention further relates to anti viral agents comprised HIV viral fusion inhibitors and an Fc fragment of an immunoglobulin. The invention also relates to methods of treating a viral infection, including HIV infection.
Owner:HEHIR CRISTINA +5
Methods, compositions, formulations, and uses of cellulose and acrylic-based polymers
InactiveUS20050244365A1Easy to chargeLow pKaAntibacterial agentsCosmetic preparationsDisinfectantReverse transcriptase
Compositions, formulations, and methods for the treatment or prevention, or decreasing the frequency of transmission of a virus (such as human immunodeficiency virus type 1 (HIV-1), Herpes Simplex virus type 1 (HSV1), or Herpes Simplex Virus Type 2 (HSV2), or other virus), or a bacterial infection (such as Trichomonas vaginalis, Neisseris gonorrhoeae Haemopholus ducreyl, or Chlamydia trachomatis, or other bacterial species), or a fungal infection, using an anionic cellulose- or acrylic-based oligomer, polymer, or copolymer. The present invention also includes administering a therapeutically effective amount of said oligomer, polymer, or copolymer, or a pharmaceutically acceptable salt thereof, or with a pharmaceutically acceptable carrier or diluent, thereof. The invention relies on the unique biochemical substitution of the cellulose or acrylic backbone such that the resultant molecule can remain molecularly dispersed in solution (or gel or other formulation) and mostly dissociated over a wide range of physiological microenvironments, such as the low pH found within the vaginal lumen, preferably from a pH of 14 to below 3.5. These specific substitutions also impart on the resultant molecule potent antiviral, anti-bacterial, and anti-fungal properties. In addition, these compositions can be used as general disinfectants for human use such as in contact lens solutions, mouthwashes, toothpastes, suppositories, or as more generalized disinfectants found in soaps, household cleaning products, paints, water treatments modalities, or can be incorporated into cosmetic, and can be used as vehicles for drug delivery, an adjuvant in a therapeutic formulation, or as a preservative. These compounds can be delivered in a liquid or solid dosage form and can be incorporated into barrier devices such as condoms, diaphragms, or cervical caps, to help prevent the transmission of STDs. The compounds of this invention can also be used in combination therapies with other classes of antiviral, antibacterial, or antifungal agent having similar or differing mechanisms of action including, but not limited to, anionic or cationic polymers, copolymers, or oligomers, surfactants, protease inhibitors, DNA or RNA polymerase inhibitors (including reverse transcriptase inhibitors), fusion inhibitors, cell wall biosynthesis inhibitors, integrase inhibitors, or virus or bacterial attachment inhibitors.
Owner:NOVAFLUX INC +1
Treatment of HIV and aids using probiotic lactobacillus reuteri
InactiveUS20100143305A1Reducing and preventing depletionReducing and preventing HIV replicationBiocidePeptide/protein ingredientsImmunodeficiency virusHuman immunodeficiency
A method for treating or preventing a Human Immunodeficiency Virus (HIV) infection, or treating or preventing Acquired Human Immunodeficiency Syndrome (AIDS), in a subject in need thereof, is disclosed. The method involves colonizing a genetically modified probiotic Lactobacillus reuteri RC-14 in the gastrointestinal tract of a subject, wherein the probiotic is able to secrete two or more fusion inhibitors that decrease or prevent HIV production and CD4+T cell depletion in the gastrointestinal tract.
Owner:LEMKE JAMES ALLEN
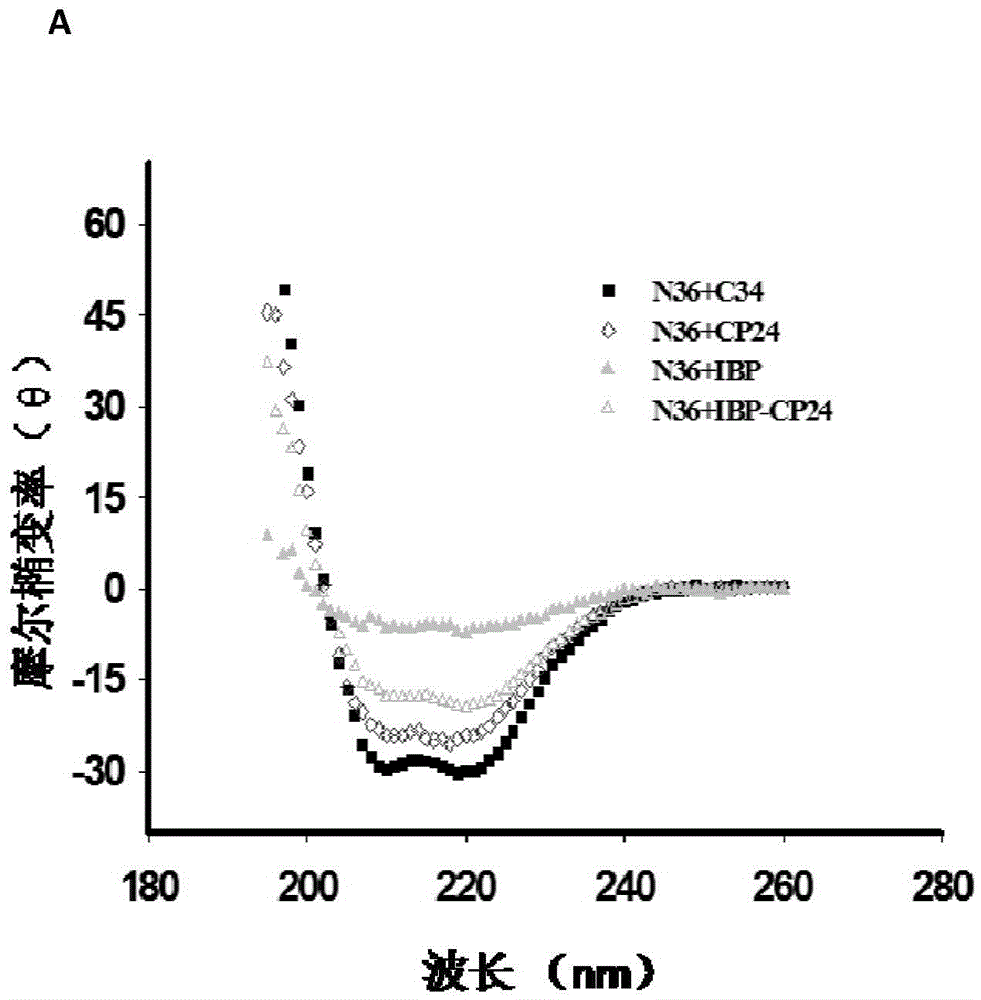
Multimerised HIV fusion inhibitors
InactiveUS20050202043A1Improve isolationHigh protein yieldPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunodeficiency virusTherapy HIV
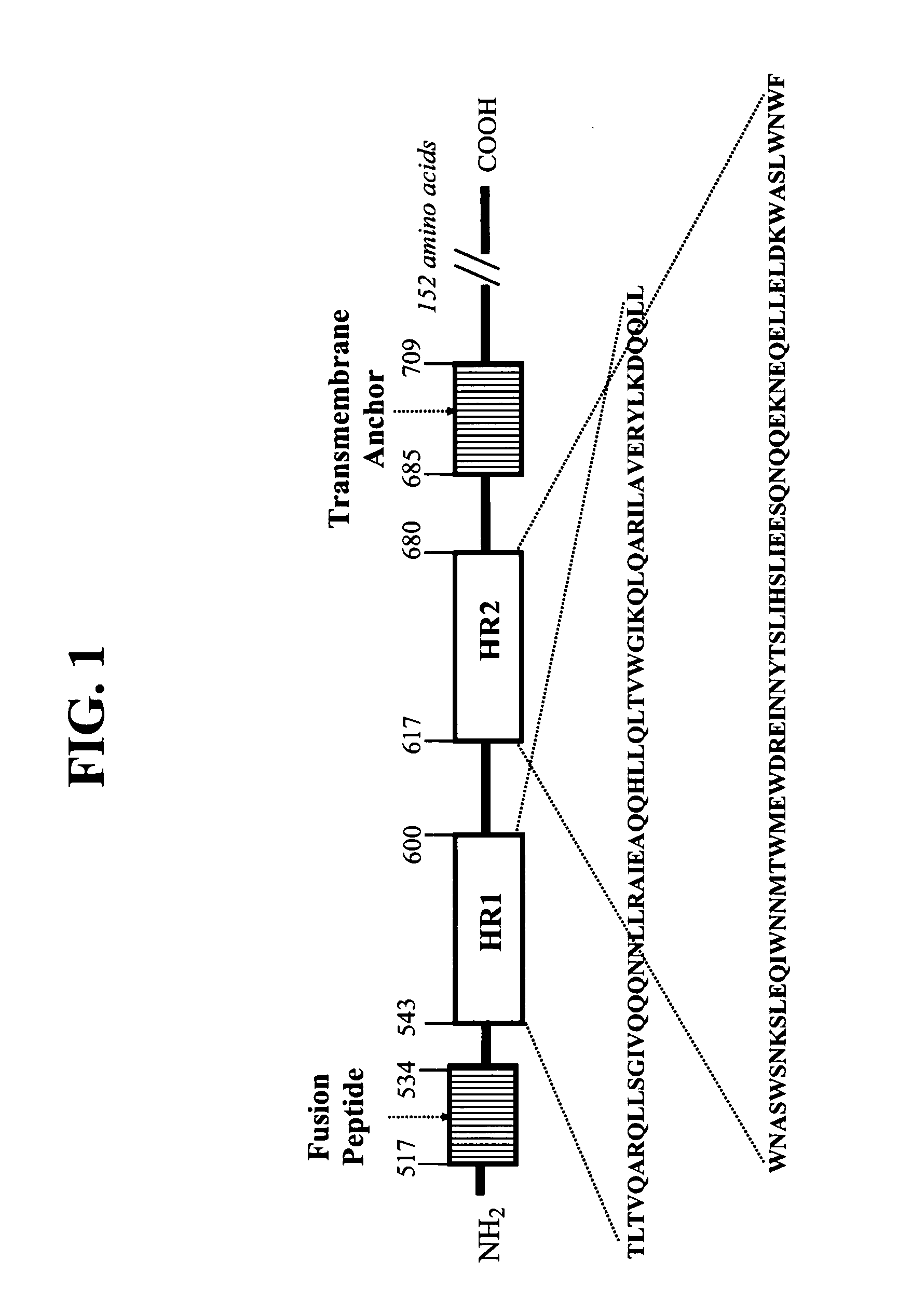
There are provided multimeric fusion proteins exhibiting anti-viral activity. The fusion proteins comprise the HR2 region of the ectodomain of the human immunodeficiency virus gp41 protein which is fused to a multimerisation domain peptide such as a trimerisation domain derived from tetranectin. The multimerised fusion proteins may be used as HIV fusion inhibitors in the treatment of AIDS.
Owner:BOREAN PHARMA APS
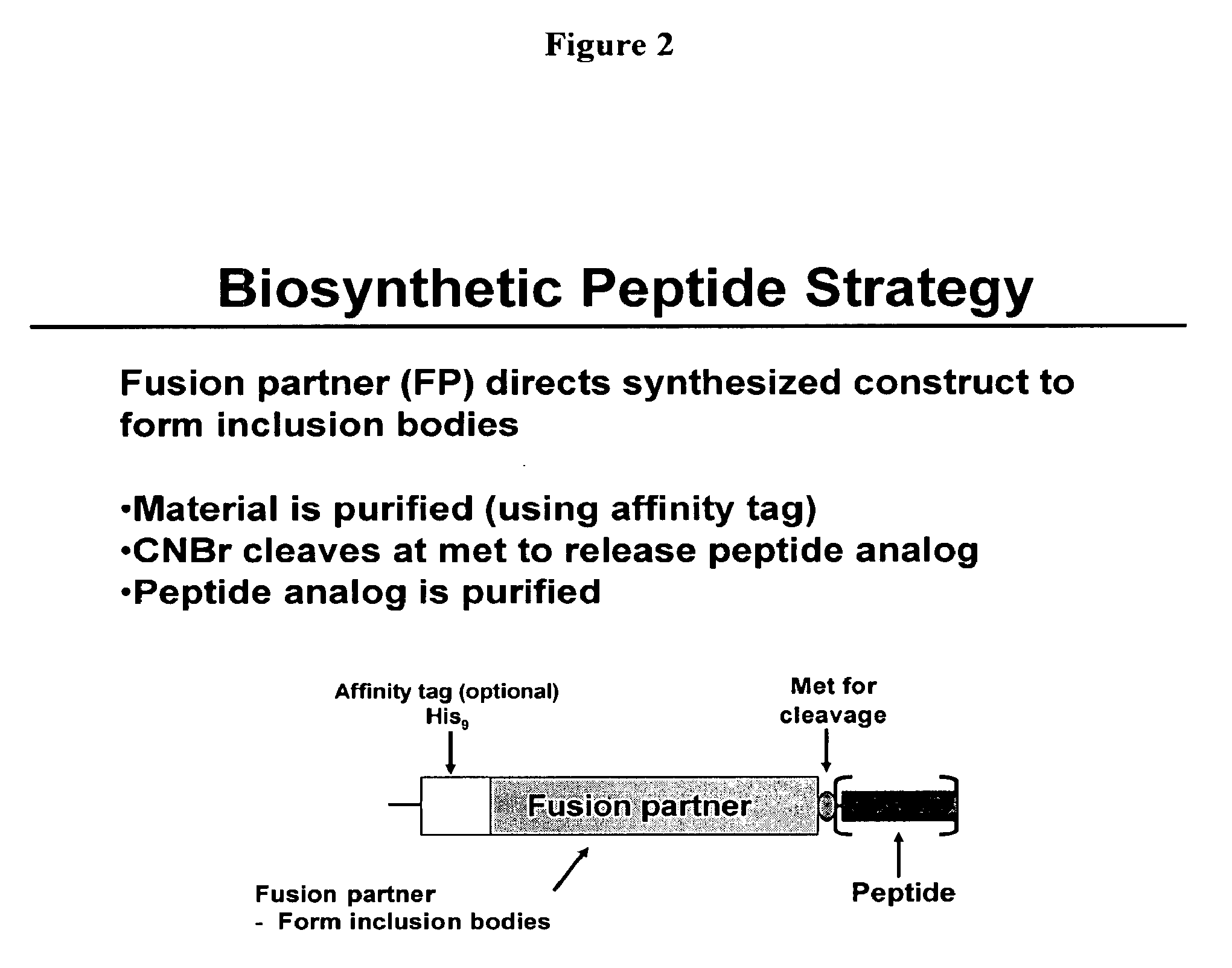
Biosynthetic Polypeptide Fusion Inhibitors
InactiveUS20080113408A1Extended half-lifeImprove stabilitySugar derivativesPeptide/protein ingredientsFusion inhibitorBiosynthesis
Owner:AMBRX
Biosynthetic Polypeptide Fusion Inhibitors
InactiveUS20080112943A1Extended half-lifeImprove stabilitySugar derivativesPeptide/protein ingredientsFusion inhibitorBiosynthesis
Owner:AMBRX
Process for manufacturing magnetic material, magnetic material and high density magnetic recording medium
InactiveUS20060204793A1High densityEfficiently obtainedNanomagnetismInorganic material magnetismHigh densityFerromagnetic order
A process for manufacturing a magnetic material, comprising: coating a nanoparticle dispersion containing alloy nanoparticles capable of forming a ferromagnetic ordered alloy phase, and a fusion inhibitor on a support to form a coated film of a nanoparticle magnetic layer, and heat-treating the coated film to ferromagnetize the alloy nanoparticles. Further, a magnetic material comprising a support and a nanoparticle magnetic layer of a nanoparticle dispersion coated thereon, wherein the nanoparticle dispersion comprises alloy nanoparticles capable of forming a ferromagnetic ordered alloy phase and a fusion inhibitor, is provided.
Owner:FUJIFILM HLDG CORP +1
Flavivirus fusion inhibitors
InactiveUS20050271677A1Reduce HIV- loadImprove developmentSsRNA viruses positive-sensePeptide/protein ingredientsPestivirusVirosome
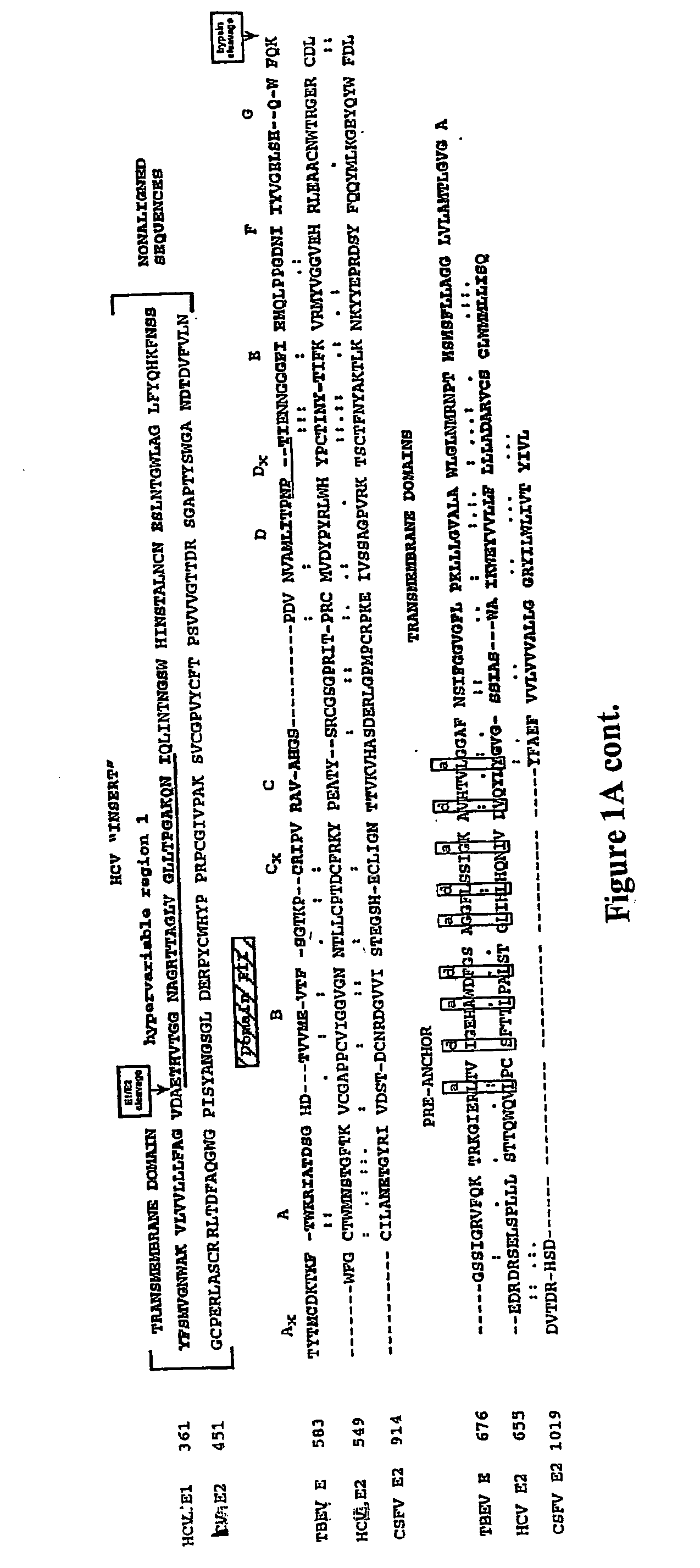
The present invention relates to peptides and methods of inhibiting fusion between the virion envelope of Flaviviruses and membranes of the target cell, the process that delivers the viral genome into the cell cytoplasm. The invention provides for methods which employ peptides or peptide derivatives to inhibit Flavivirus:cell fusion. The present invention is based in part on the discovery that E1 envelope glycoprotein of hepaciviruses and E2 envelope glycoprotein of pestivirus have previously undescribed structures, truncated class II fusion proteins. The present invention provides peptides and methods of treatment and prophylaxis of diseases induced by Flaviviruses.
Owner:TULANE EDUCATIONAL FUND +1
Pharmaceutical composition for improved administration of HIV gp41-derived peptides, and its use in therapy
ActiveUS7045552B2Reduce refactoring timeLow viscosityBiocidePeptide/protein ingredientsPolyolHiv infected
Provided is a pharmaceutical composition comprising a solution comprised of synthetic peptide in a final concentration of not less than 70 mg / ml in admixture with a polyol; wherein the synthetic peptide is an HIV fusion inhibitor, and wherein the polyol is in a final concentration of no less than 5 weight % and no more than 75 weight % of the pharmaceutical composition. Also provided is a synthetic peptide-containing pharmaceutical composition as a unit dose comprising an aqueous formulation comprised of synthetic peptide in a final concentration of not less than 70 mg / ml in admixture with a polyol; wherein the synthetic peptide is an HIV fusion inhibitor, and wherein the polyol is in a final concentration of no less than 5 weight % and no more than 75 weight % of the pharmaceutical composition. Further provided is a method of treating HIV infection by administering to an HIV-infected individual a pharmaceutical composition according to the present invention.
Owner:TRIMERIS
Synergistic compositions for the prevention and treatment of acquired immunodeficiency syndrome
InactiveUS20060121480A1Good curative effectLess drugPeptide/protein ingredientsMicrobiological testing/measurementAcquired immunodeficiency syndrome (AIDS)Synergistic combination
A method for preventing infection of helper T and other target cells by human immunodeficiency virus type 1 (“HIV-1”) and for preventing or treating acquired immunodeficiency syndrome (“AIDS”) by exposing target cells to a synergistic combination of at least one attachment inhibitor and at least one fusion inhibitor. The attachment inhibitors are compounds that bind to the CD4 receptor on target cells or that bind to gp120 on HIV-1, e.g., antibodies, and the fusion inhibitors compounds that interact with gp41 to inhibit or prevent its interaction with target cells, e.g., pentafuside.
Owner:GENENTECH INC
Long lasting fusion peptide inhibitors of viral infection
InactiveUS20080199483A1Reduce sensitivityImprove in vivo stabilityPeptide/protein ingredientsVirus peptidesBlood componentHalf-life
Peptides exhibiting anti-viral and anti-fusogenic activity are modified to provide greater stability and improved half-life in vivo. The selected peptides include fusion inhibitors DP178 and DP107 and related peptides and analogs thereof. The modified peptides are capable of forming covalent bonds with one or more blood components, preferably a mobile blood component.
Owner:CONJUCHEM
Inhibitors of HIV membrane fusion
InactiveUS20050221294A1Improve stabilityImprove solubilityCompound screeningApoptosis detectionCoiled coilMembrane configuration
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
Inhibitors of Infection
InactiveUS20090011000A1Produce significantLow protein yieldSsRNA viruses positive-sensePeptide/protein ingredientsADAMTS ProteinsTransmembrane domain
Fusion inhibitor peptides are provided comprising a sequence derived from an HR2 domain. In preferred embodiments, the peptides are capable of oligomerization. Also provided are nucleic acids encoding the peptides, vectors comprising the nucleic acids and host cells transformed with the vectors. The peptides may be used as medicaments. Also provided are methods for expressing a protein comprising one or more transmembrane domain(s) in an expression system. The methods comprise fusing a sequence encoding an 18-mer peptide, or a functional equivalent thereof, to a gene encoding the protein and expressing the resultant gene-fusion product in an expression system.
Owner:HINZ ANDREAS +2
Compound fusion inhibitor capable of increasing fusion temperature of coal ash with low ash fusion point
The invention discloses a compound fusion inhibitor capable of increasing fusion temperature of coal ash with a low ash fusion point and belongs to the technical field of coal gasification improvement. The compound fusion inhibitor comprises raw materials in parts by weight as follows: 1-30 parts of calcium-based flux and 1-30 parts of aluminium oxide. The calcium-based flux is doped into the fusion inhibitor aluminium oxide, so that the fusion point of the coal ash can be increased effectively, coal with low ash fusion point can meet gasification requirements, viscosity-temperature characteristics can be improved, gasification efficiency is increased, cost can be reduced, and the coal with low ash fusion point can be applied better.
Owner:FUZHOU UNIV
Long lasting fusion peptide inhibitors of viral infection
InactiveUS20080176794A1Improve in vivo stabilityReduce sensitivityOrganic active ingredientsBiocideBlood componentHalf-life
Peptides exhibiting anti-viral and anti-fusogenic activity are modified to provide greater stability and improved half-life in vivo. The selected peptides include fusion inhibitors DP178 and DP107 and related peptides and analogs thereof. The modified peptides are capable of forming covalent bonds with one or more blood components, preferably a mobile blood component.
Owner:CONJUCHEM
HIV fusion inhibitor peptides with improved biological properties
ActiveUS20070179278A1More potencySugar derivativesPeptide/protein ingredientsBiological propertyAmino acid
Provided is an HIV fusion inhibitor peptide having an amino acid sequence of any one of SEQ ID NO:9, SEQ ID NO:10, SEQ ID NO:11, SEQ ID NO:12, SEQ ID NO:13, SEQ ID NO:14, or SEQ ID NO:15; and provided is a pharmaceutical composition comprising a HIV fusion inhibitor peptide and one or more of a pharmaceutically acceptable carrier and macromolecular carrier, and uses and methods of treatment provided by these compositions.
Owner:TRIMERIS
Mutant proteins of the f protein of piv-5 and piv-2
InactiveUS20110318355A1Large capacityHigh autonomyOrganic active ingredientsVirusesProgenitorMutated protein
The present application concerns mutant proteins of the fusion protein (F protein) of the parainfluenza virus (PIV) which are currently indexed as type 5 PIV (PIV-5 or PIV5) and type 2 PIV (PIV-2 or PIV2). The present application concerns products deriving therefrom, such as: nucleic acids, vectors, cells, fusion inhibitors of the antibody, aptamer, interfering RNA type; myelomas, hybridomas; stem and progenitor cells. The present application also concerns mutant proteins and products derived therefrom for use in medical and biotechnological applications.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Biological preparation for preventing and controlling human respiratory syncytial virus infection and preparation method
ActiveCN104353063AImprove stabilityLow costPeptide/protein ingredientsAntiviralsHuman respiratory virusViral infection
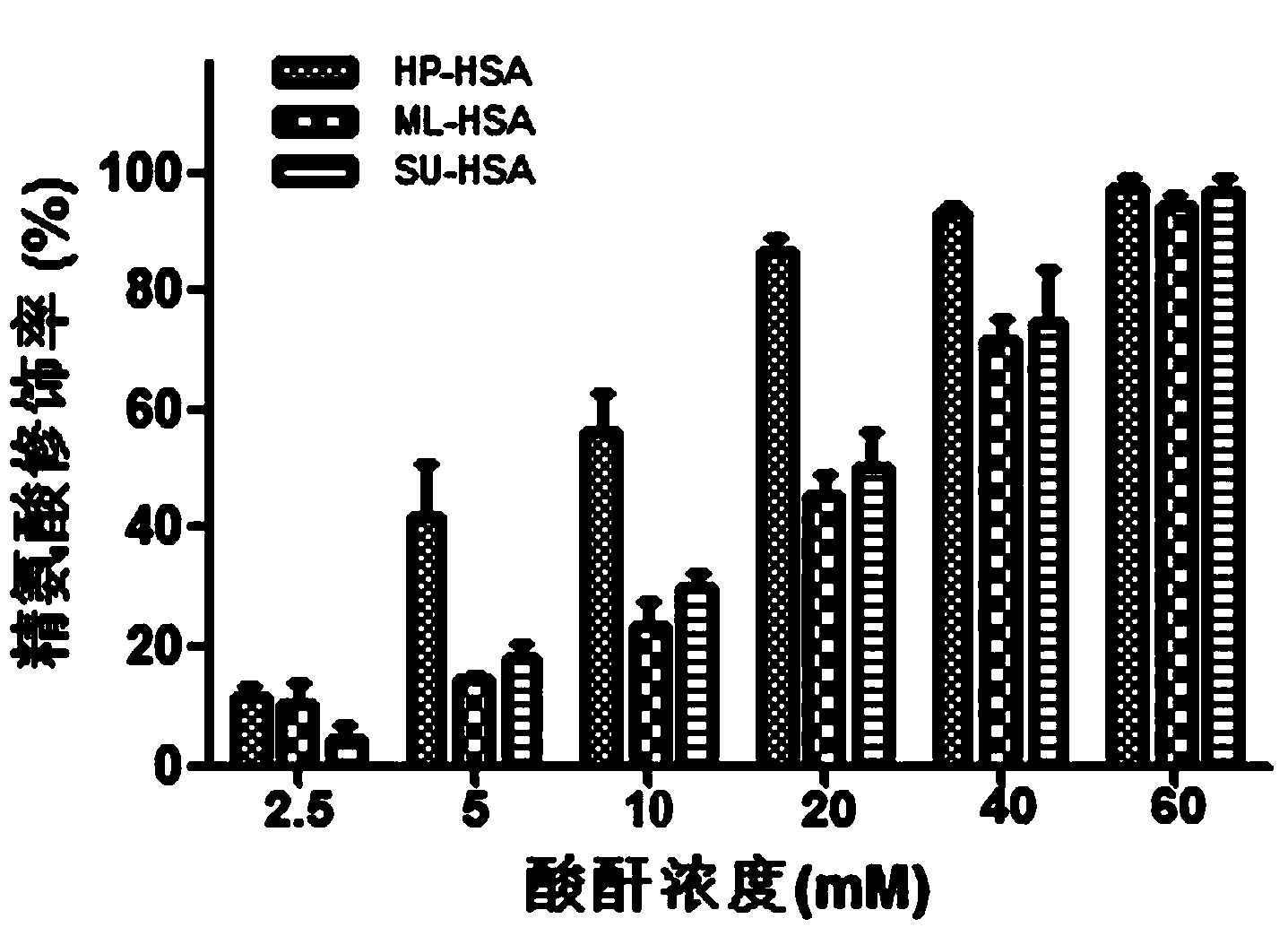
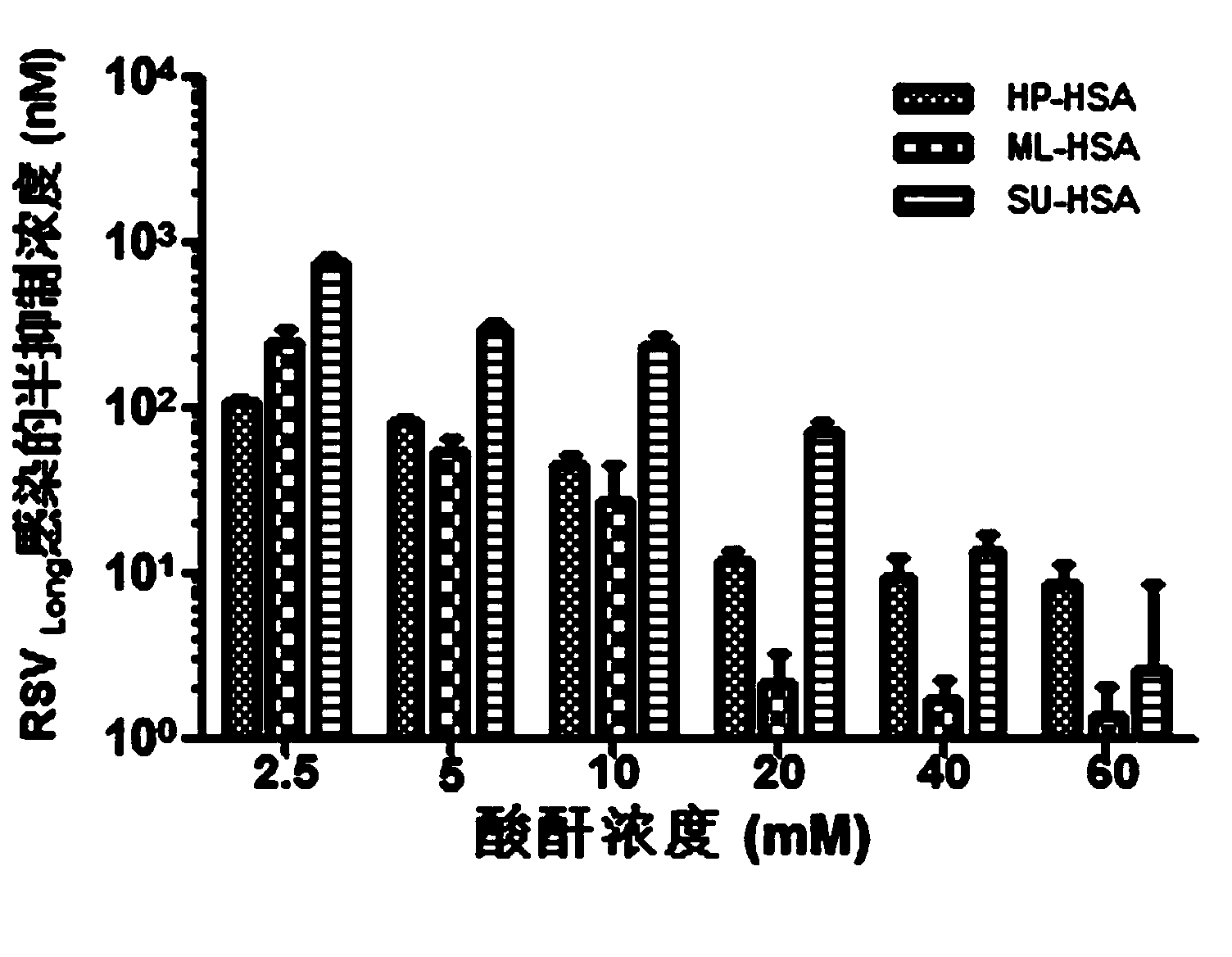
The invention relates to a novel antiviral biological preparation in the technical field of medicines, in particular to a biological preparation for preventing and controlling human respiratory syncytial virus infection and a preparation method. The biological preparation adopts globulin virus entry or fusion inhibitors. The biological preparation is applied to the first stage that virus invades target cells, and blocks the infection of virus to the cells, so as to achieve the effect of preventing and controlling the virus. The biological preparation adopts active anhydride to modify separated and purified amino acid with positive charge on the surface, thereby having the function of preventing the human RSV (respiratory syncytial virus) from entering and infecting the target cells.
Owner:SHANXI JINBO BIO PHARMA CO LTD
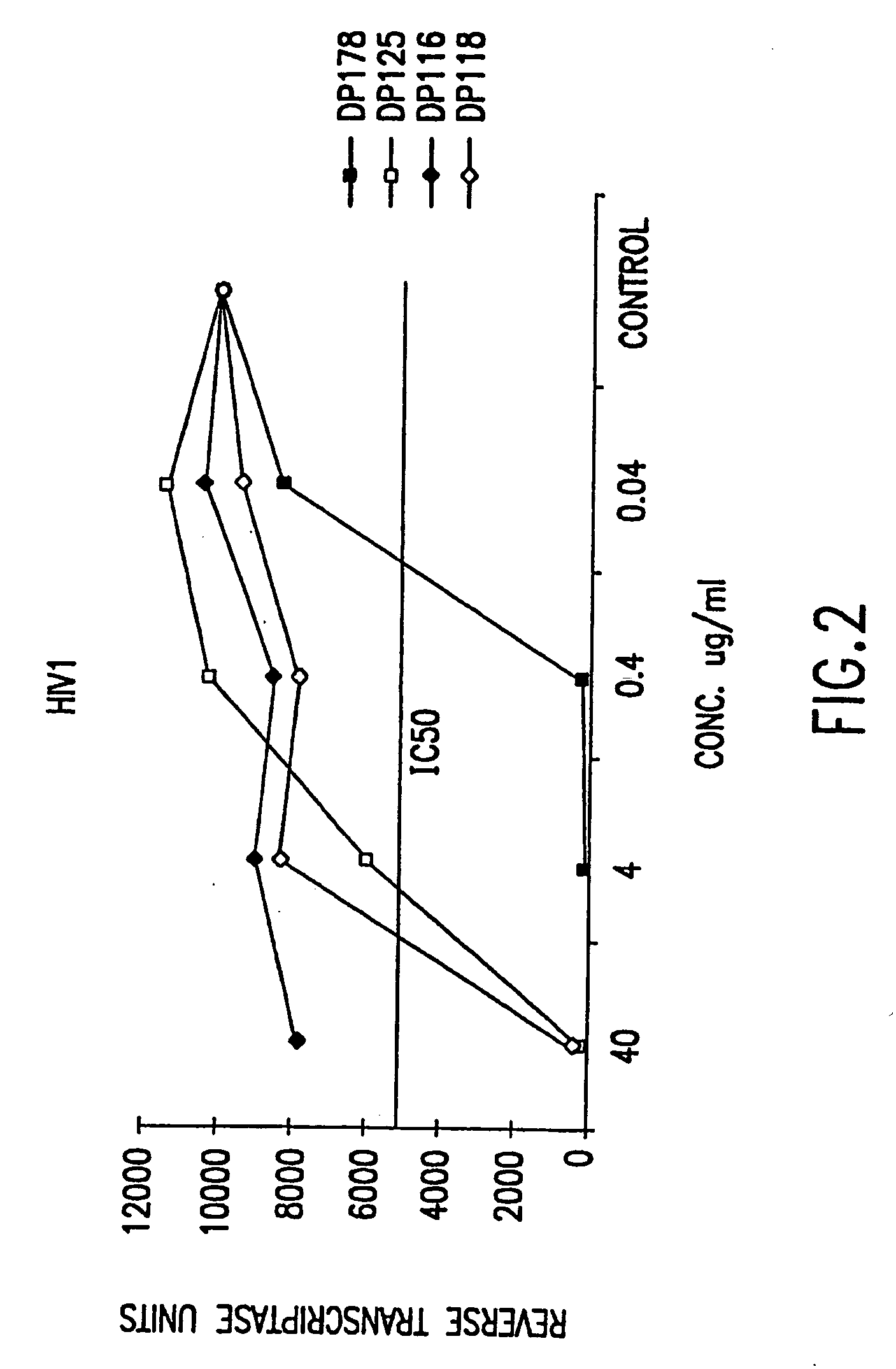
Nucleic acids encoding DP-178 and other viral fusion inhibitor peptides useful for treating aids
InactiveUS20070202127A1Avoid virus infectionSsRNA viruses negative-sensePeptide/protein ingredientsTherapy HIVUninfected cell
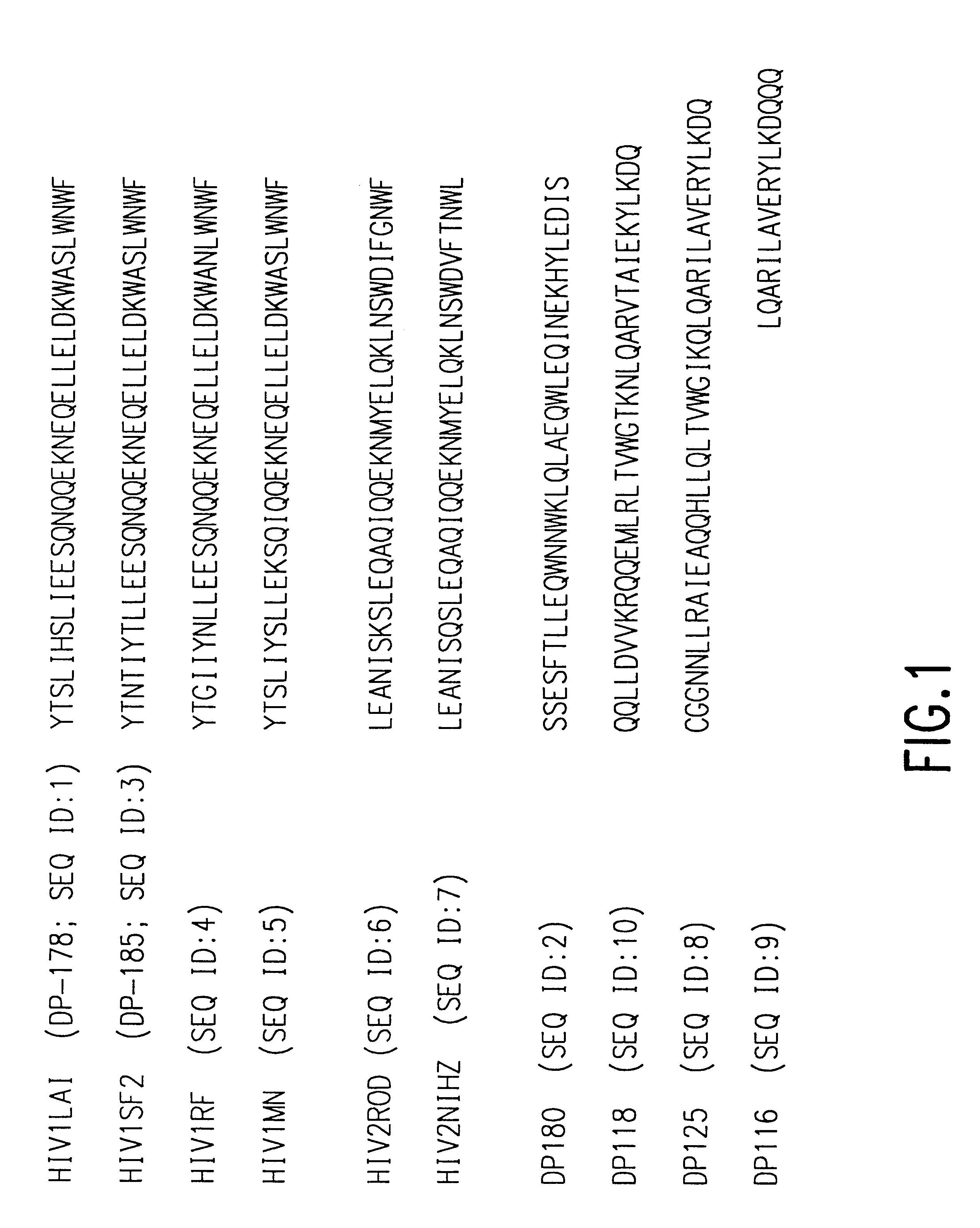
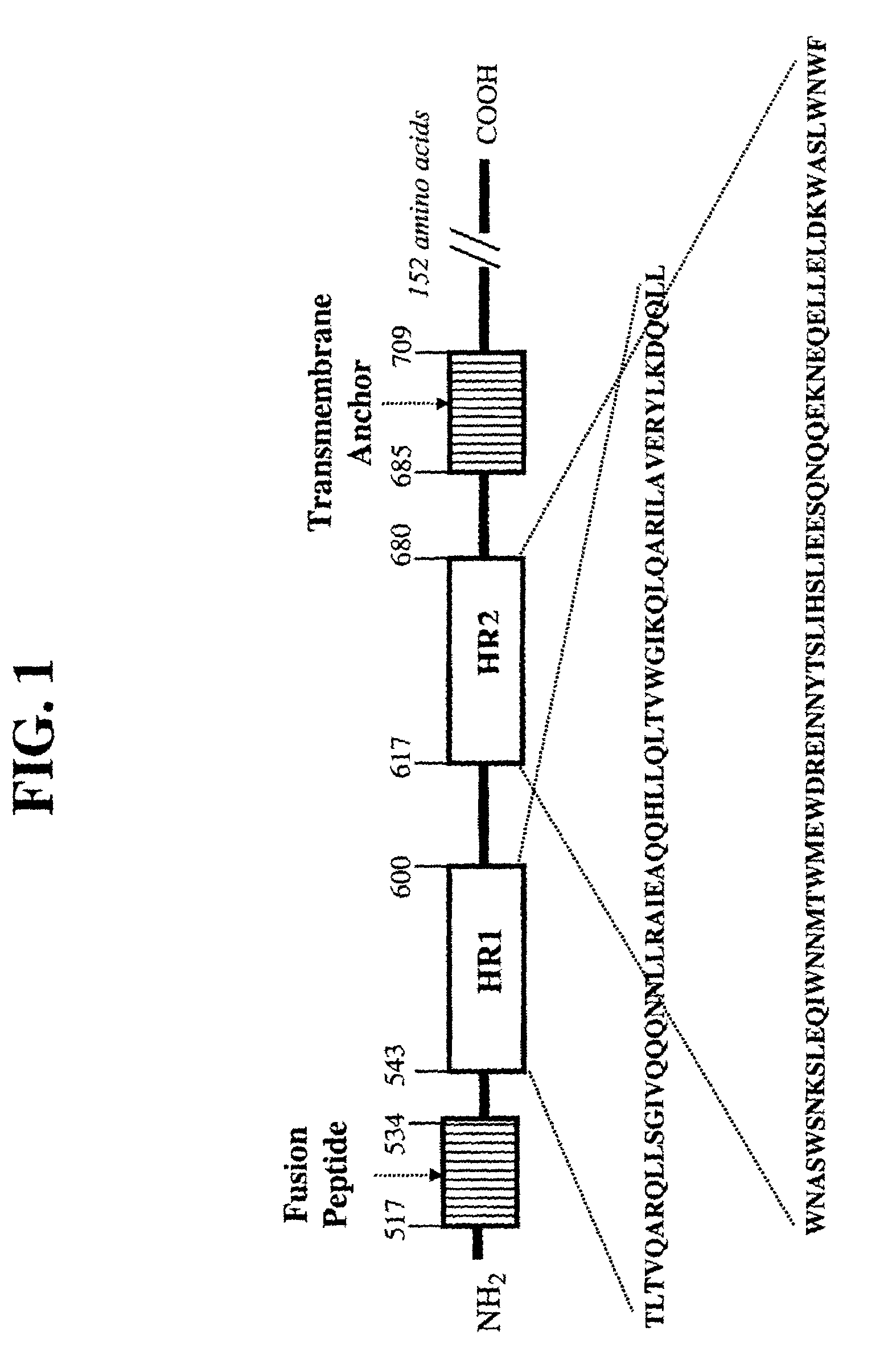
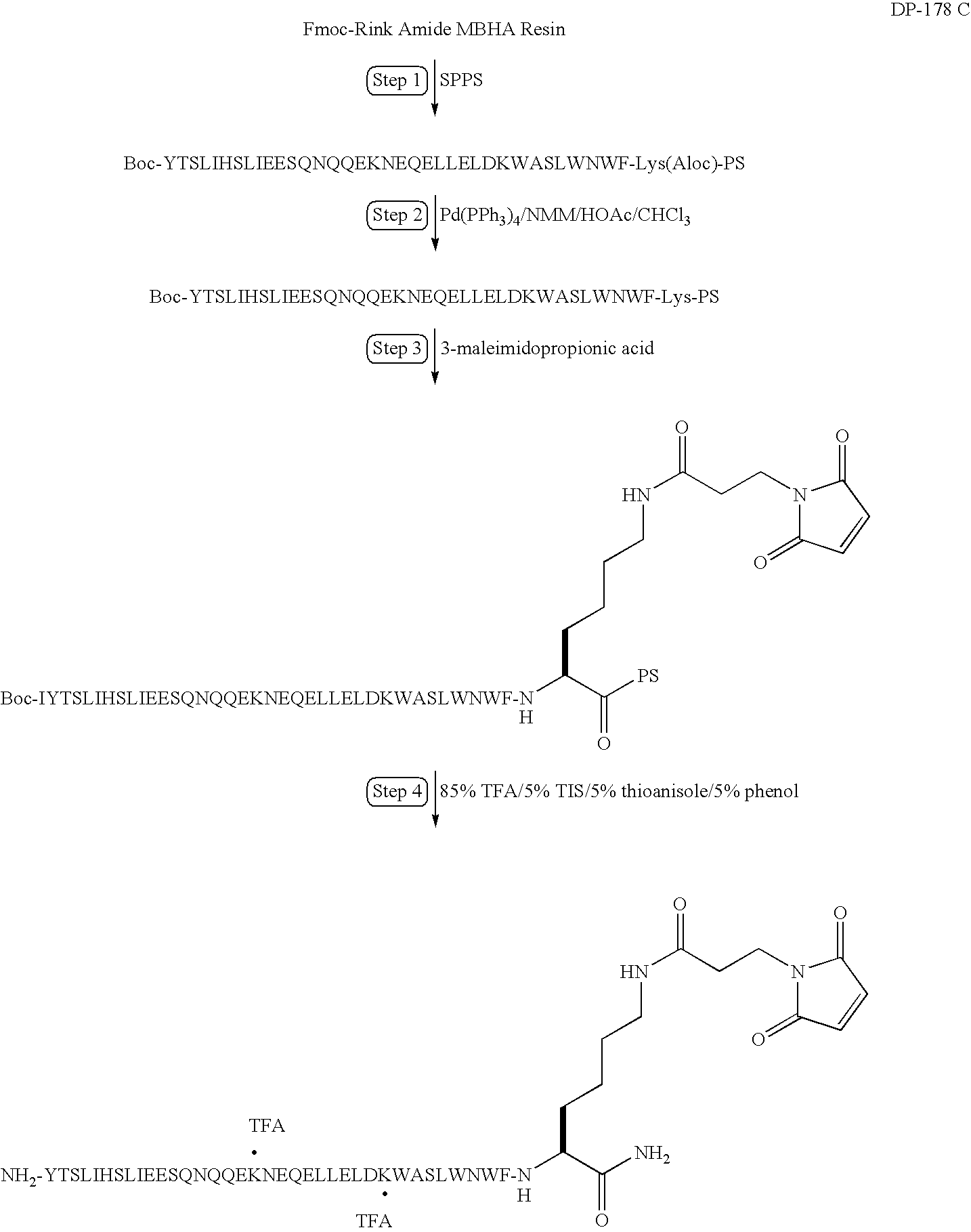
The present invention relates to peptides which exhibit potent anti-retroviral activity. The peptides of the invention comprise DP178 (SEQ ID:1) peptide corresponding to amino acids 638 to 673 of the HIV-1LAI gp41 protein, and fragments, analogs and homologs of DP178. The invention further relates to the uses of such peptides as inhibitory of human and non-human retroviral, especially HIV, transmission to uninfected cells.
Owner:DUKE UNIV +1
Long-acting HIV fusion inhibitor and application thereof
ActiveCN105646717APromote research and developmentExtended half-lifePeptide/protein ingredientsGenetic material ingredientsHalf-lifeTherapy HIV
Owner:FUDAN UNIV
Long lasting fusion peptide inhibitors of viral infection
Peptides exhibiting anti-viral and anti-fusogenic activity are modified to provide greater stability and improved half-life in vivo. The selected peptides include fusion inhibitors DP178 and DP107 and related peptides and analogs thereof. The modified peptides are capable of forming covalent bonds with one or more blood components, preferably a mobile blood component.
Owner:CONJUCHEM BIOTECH INC
Method of treating asthma with antiviral agents
InactiveCN102573886AOrganic active ingredientsPeptide/protein ingredientsNucleoside Reverse Transcriptase InhibitorInhalation
Owner:MUTUAL PHARMA CO INC
HIV-1 membrane fusion inhibitor and application thereof
InactiveCN101914143ADoes not cause significant degradationQuick clearFungiBacteriaViral envelopeAmino acid
The invention relates to a separated peptide for inhibiting HIV-1 mediated membrane fusion, which is characterized in that the peptide comprises an amino acid sequence of WGRLEGRRT (SEQ ID NO: 3). The peptide can react mutually with gp41, effectively prevent cell fusion mediated by viral envelope protein, and neutralize HIV-1.
Owner:TSINGHUA UNIV
Novel druggable regions in the dengue virus envelope glycoprotein and methods of using the same
The present invention relates to novel druggable regions discovered in dengue virus envelope glycoprotein, or dengue virus E protein, which is a class II viral E protein. The present invention further relates to methods of using the druggable regions to screen potential candidate therapeutics for diseases caused by viruses having class II E proteins, e.g. viral fusion inhibitors.
Owner:CHILDRENS MEDICAL CENT CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com