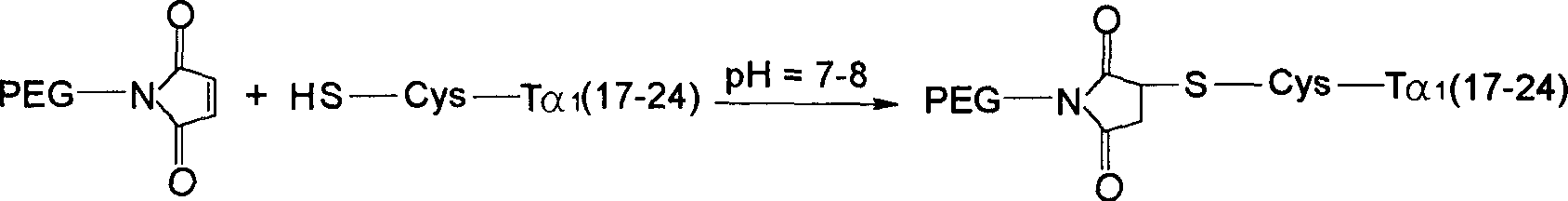Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "IMMUNOLOGIC DEFICIENCY" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunodeficiency (or immune deficiency) is a state in which the immune system's ability to fight infectious disease and cancer is compromised or entirely absent. Most cases of immunodeficiency are acquired ("secondary") due to extrinsic factors that affect the patient's immune system.
HIV antibody and antigen combined rapid detection reagent kit
ActiveCN101266246AImprove the detection rateShorten the windowMaterial analysisDiseaseDirect observation
The invention relates to biology applied technology field, especially relates to a immune chromatography assay for quickly detecting the human immunologic deficiency disease (HIV) antibody and antigen. The assay comprises two independent test papers A and B and plastic case C for storing the test paper, wherein the test paper A is used for detecting the HIV antibody and the test paper B is used for detecting the HIV p24 antigen. The test paper A and test paper B are parallely encased in the plastic case to constitute the assay. On detecting, the detected sample is added on the sample pad of the test paler and the immunoreaction result is directly observed to perform detection. The assay is used for screening or clinical diagnosis of the HIV infection and at the same time the HIV antigen and antibody are detected, the simpler antibody detection can effectively reduce the window phase for detecting the HIV, with features of quick reaction, easy operation, economy and practicality, suitable for insitu detecting.
Owner:天津中新科炬生物制药股份有限公司
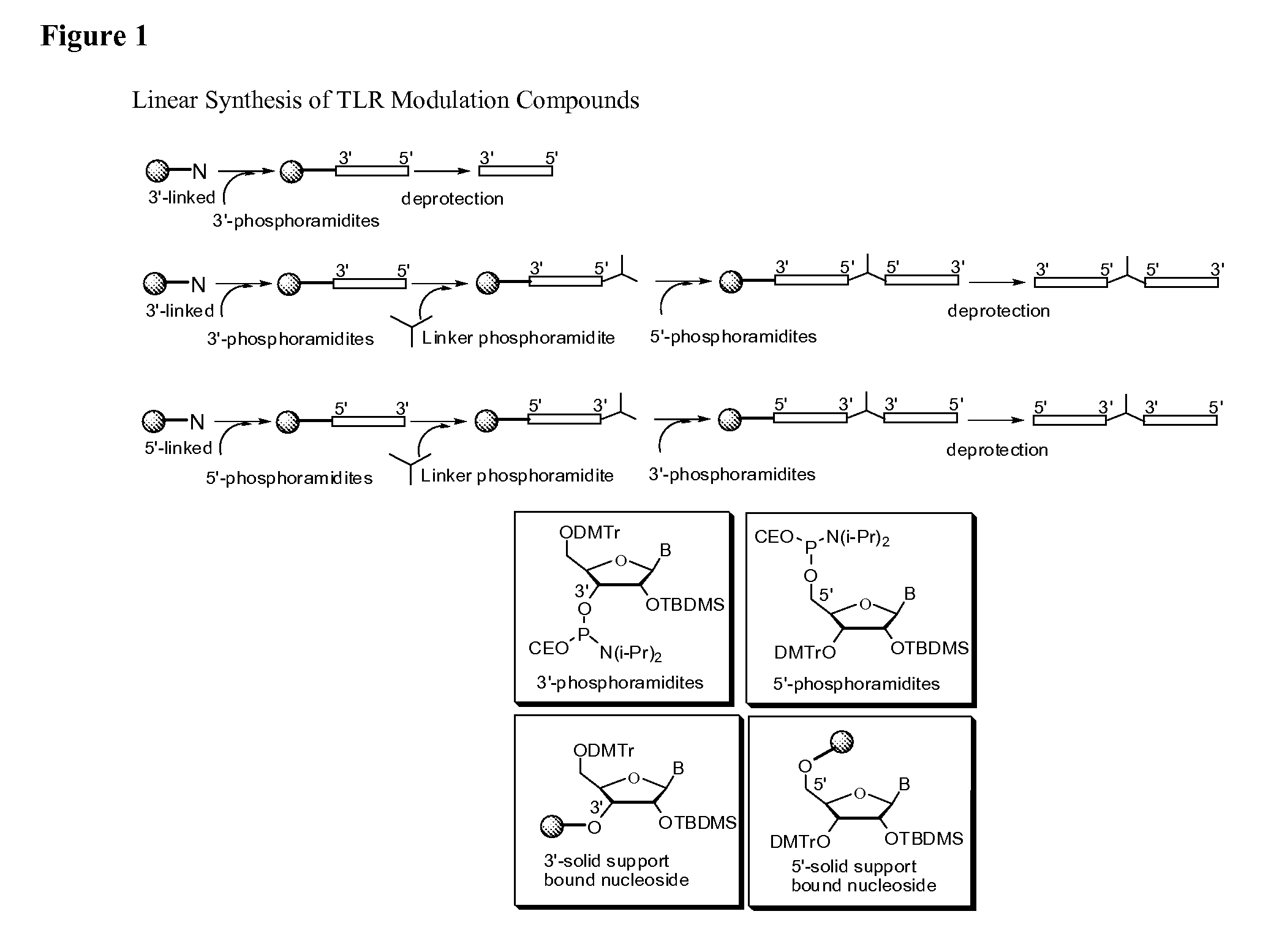
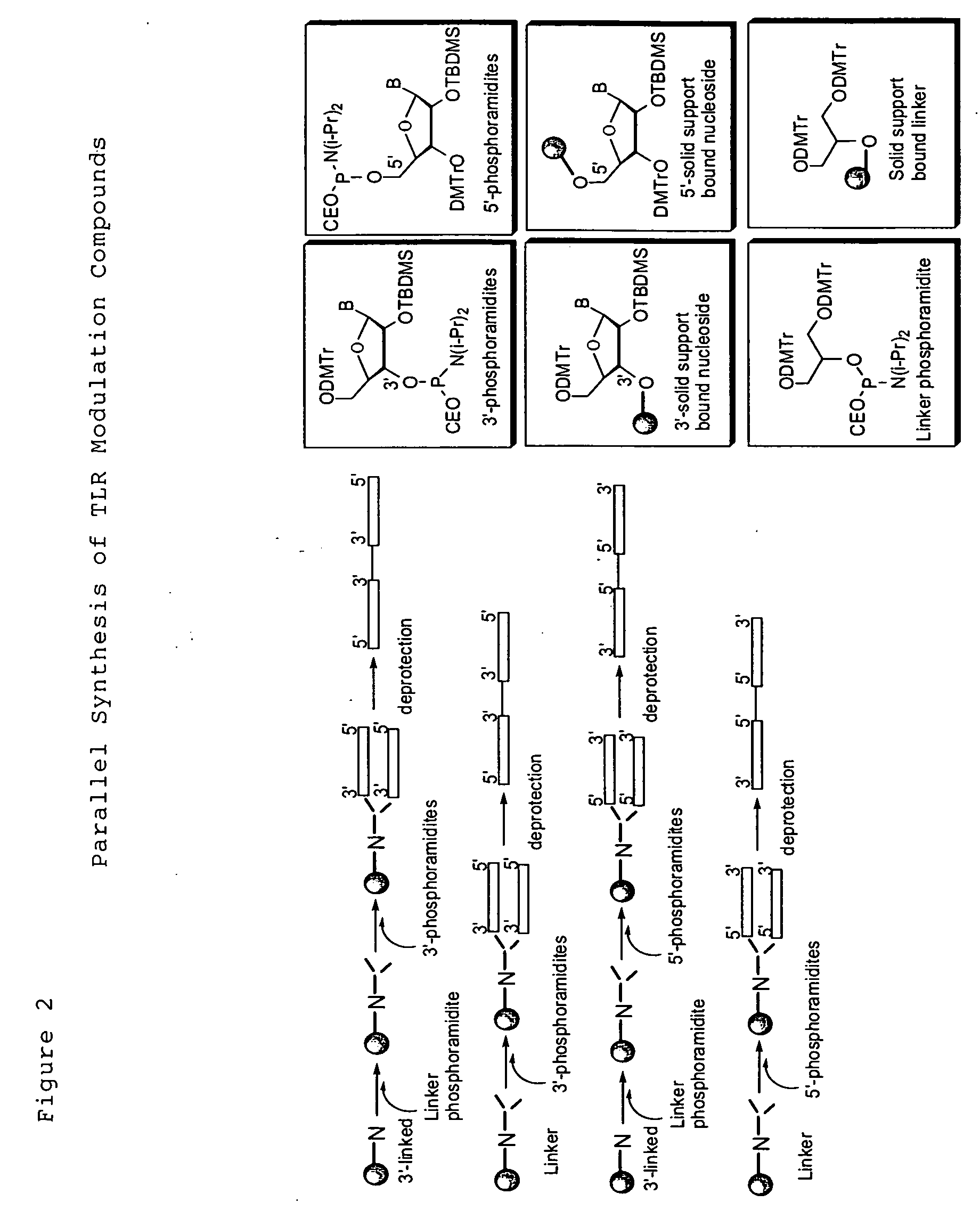
Toll like receptor modulators
ActiveUS20090053148A1Prevent diseaseLower immune responseSenses disorderNervous disorderInflammatory Bowel DiseasesAutoimmune responses
The invention relates to TLR9 antagonist compounds and their therapeutic or prophylactic use. The invention provides novel immune regulatory oligonucleotides and immunomers as antagonist of TLRs and methods of use thereof. These immune regulatory oligonucleotides have unique sequences that suppress, without completely ablating, TLR-mediated signaling in response to a TLR ligand or TLR signaling agonist. The methods may have use in the prevention and treatment of autoimmunity, inflammation, inflammatory bowel disease, lupus, allergy, asthma, infection, sepsis, cancer and immunodeficiency.
Owner:IDERA PHARMA INC
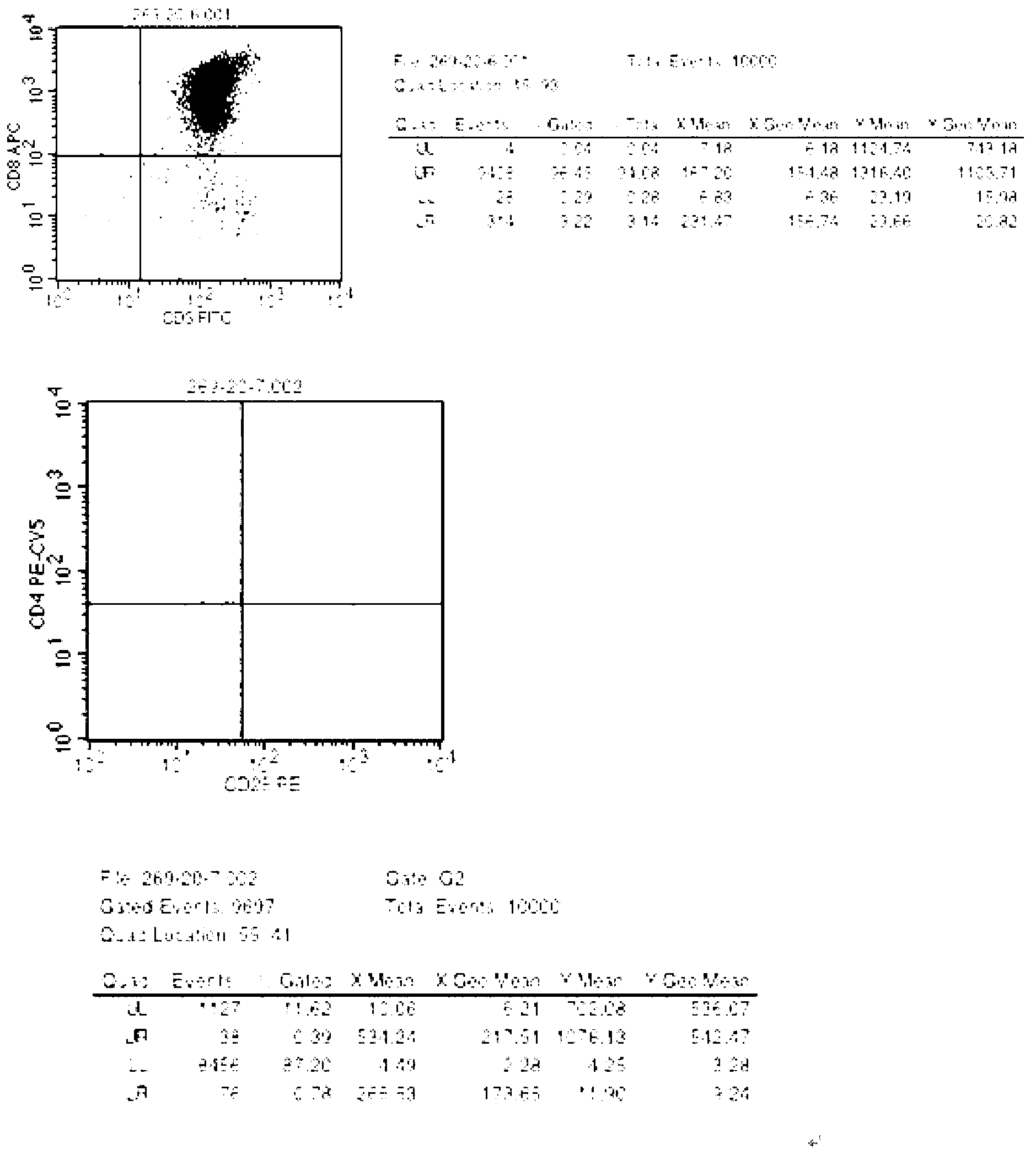
Amplifying, freezing and storing and recovering method of activated lymphocyte with CD3+CD8+as major
InactiveCN102839153ASolve the problem of multiple blood collectionHigh purityDead animal preservationBlood/immune system cellsPatient needT lymphocyte
The invention discloses culturing, freezing and recovering methods of an activated lymphocyte with CD3+CD8+as major, which can solve problems that a patient needs carrying out blood sampling for many times caused by continuously utilizing the activated lymphocyte with CD3+CD8+as major. The method comprises the following steps of: (1) contacting the extracted lymphocyte of the peripheral blood with IL-2, IL-15, an anti-CD3 antibody and an anti-CD28 antibody, so as to amplify the activated lymphocyte with CD3+CD8+as major; (2) freezing and storing the activated lymphocyte; and (3) recovering the activated lymphocyte. The activated lymphocyte cultured via the method disclosed by the invention has clear components, and comprises few CD4+CD25+Treg cells and more CD8+T lymphocyte; feedback time and frequency of the activated lymphocyte can be adjusted according to other treatments for a patient, such as a radiotherapy or a chemotherapy, so that diseases, such as tumor, infectious diseases and immunodeficiency can be treated well.
Owner:JINAN TAISHENG BIOLOGICAL TECH CO LTD
Polypeptides from African Swine Fever virus as vaccines for preventive and therapeutic use
InactiveUS20080131449A1Effective preventionEffective treatmentPeptide/protein ingredientsViral antigen ingredientsMammalAfrican swine fever
The present invention generally relates to the use of selected polypeptides from African Swine Fever virus for the prevention and therapy of African Swine Fever infections as well as other infections, including immune deficiencies in mammals and humans.
Owner:RATH MATTHIAS +1
Thymosin alpha 1 active segment cyclicpeptide analogue and its poly glycol derivative
The present invention relates to a kind of cyclopeptide derivative containing natural or artificial amino acid substituted active thymosin alpha-1 segemnt, and its preparation process, medicine composition and their medicines for treating or preventing diseases related to immune deficiency, hypoimmunity, etc.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Green feed additive and preparation method thereof
ActiveCN101606581APromote peristalsisGood antibacterial effectAnimal feeding stuffAccessory food factorsBiotechnologyDisease
The invention discloses a green feed additive and a preparation method thereof. The green feed additive is prepared by mixing the following components in portion by weight: 40 to 60 portions of cortex eucommiae extract, 10 to 20 portions of streptococcus faecalis powder, 30 to 40 portions of polysaccharide peptide powder and 8 to 20 portions of folium cortex eucommiae finemeal, wherein the cortex eucommiae extract at least contains 0.2 percent of chlorogenic acid; the polysaccharide peptide powder is prepared by concentrating, decoloring, precipitating with alcohol and drying the ferment liquid which is obtained by fermenting the streptococcus faecalis powder. The components of the invention are integrally applied, which can remarkably improve the function of an animal immune system, strengthen disease preventing ability of organism, and resist the intrusion of pathogen; the green feed additive contains virus resisting the immunodeficiency which can bidirectionally adjust the function of cellular immunity and greatly improve the survival rate of feeding animals; and under the conditions without hormone, the green feed additive can promote the growth and breed of animals, greatly improve the quality and taste of eggs and meat, and keep the meat quality more fresh and more close to wild taste.
Owner:SHANXI DAYU BIOLOGICAL ENG CO LTD
Cellular vaccines and immunotherapeutics and methods for their preparation
InactiveUS20050136066A1Minimal effectEnhance immune responsePeptide/protein ingredientsSnake antigen ingredientsImmunotherapeutic agentActivation cells
The present invention provides a method for enhancing the immunogenicity of weakly immunogenic or non-immunogenic cells, resulting in a cellular vaccine that can stimulate T cell activation, which in turn leads to an effective immune response. The cellular vaccines of the present invention are useful for the prevention and treatment of diseases which develop and / or persist by escaping the immune response triggered by T cell activation. Such diseases include, for example, all cancers, natural and induced immune deficiency states, and diseases caused by infections with a variety of pathogens.
Owner:SUNSHINE GUOJIAN PHARMA (SHANGHAI) CO LTD
HIV antibody immune magnetic beads rapid detection reagent kit
ActiveCN101266247AIncreased sensitivitySensitivity up toMaterial analysisDiseaseImmunodeficiency virus
The invention relates to a quick detection kit for detecting human immunologic deficiency disease (HIV) using nm immunomagnetic beads, related to biology applied technique field. The detected sample is added on the sampling pad of the test paper from the sampling hole and the sample dissolves the mark beads and chromatography is performed on the NC membrane, and then the data on the test paper isdetected using magnetic force detector in preset time and the result is decided based on the read data. The kit is used for screening or clinical diagnosis of the HIV infection with high sensitivity,specificity, quickspeed, convenience and suitable for detection on site and real time recording and storing the result.
Owner:天津中新科炬生物制药股份有限公司
Active fragment of thymosin alphal and its polyethylene glycol derivatives
The present invention relates to active fragment of natural or artificial amino acid substituted thymosin alpha-1 and its polyethylene glycol derivatives, their preparation process, the medicine composition containing them, and their application in the medicine for preventing and treating diseases related with immune deficiency and hypoimmunity, including hepatitis B, hepatitis C, malignant melanoma, non-small cell lung carcinoma, SARS, etc.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
JAK kinase inhibitor with 4-amino-(1H)-pyrazole structure and preparation method and application thereof
InactiveCN106349224AHigh yieldInhibit side effectsOrganic chemistryAntineoplastic agentsBone marrow fibrosisAbnormal tissue growth
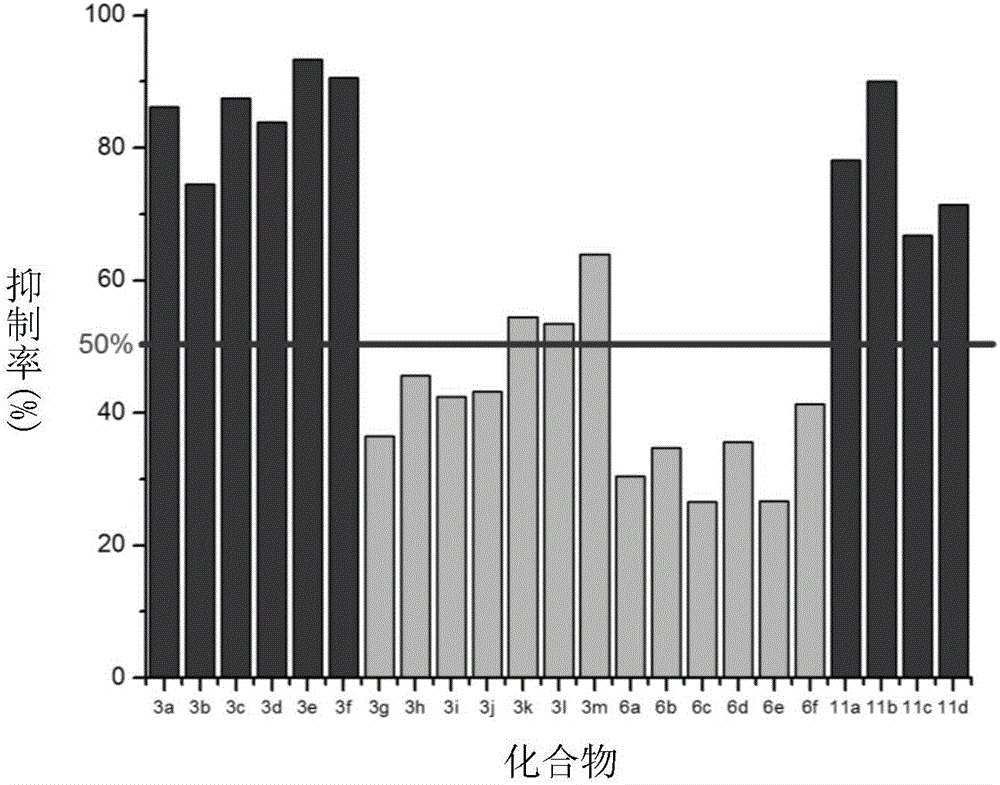
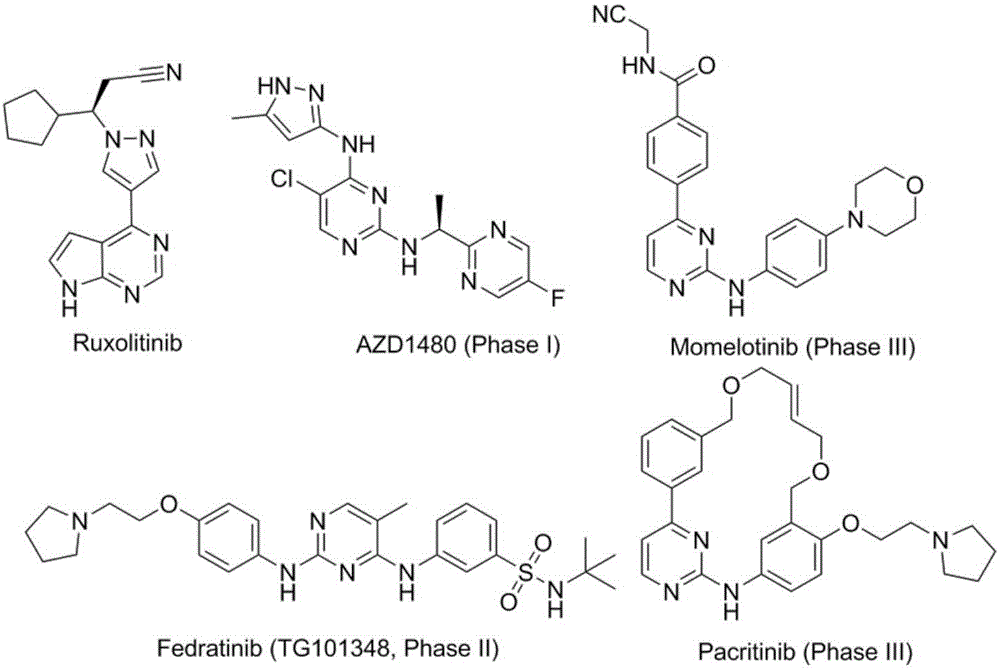
The invention discloses a JAK kinase inhibitor with 4-amino-(1H)-pyrazole structure and a preparation method and application thereof. The compound has a structure shown in general formula (I) or (II), and the invention provides a specific preparation method of the compound and application of the compound in preventing or treating inflammation, tumor and blood related diseases; the inflammation, tumor and blood related diseases include psoriasis, rheumatic arthritis, polycythemia vera, cicatricial alopecia, primary thrombocythemia, ulcerative colitis, myelofibrosis with myeloid metaplasia, severe combined immune deficiency, various leukemia, various solid tumors and the like.
Owner:SHANDONG UNIV
Method for establishing mouse transplanting tumor model with normal immunologic function
InactiveCN102125698AGenetic material ingredientsIn-vivo testing preparationsDiseaseAbnormal tissue growth
The invention relates to a method for establishing a mouse transplanting tumor model with a normal immunologic function. The mouse transplanting tumor model with the normal immunologic function is successfully established by utilizing a wild mouse as a carrier and utilizing p53- / -+S+Ras and p53- / -+V+Ras mouse tumor cells which are near-source, have clear molecular genetic background and carry conventional p53 and Ras genic mutation in the human tumor. Compared with the traditional mouse transplanting tumor model with immunodeficiency, the model provided by the invention more faithfully simulates the condition of the human tumor disease and can be used for evaluating a medicament for achieving the tumor resistant effect by improving the body immunologic function. The mouse tumor model established by the method has the advantages of short period and high repeatability. In addition, by using the mouse tumor cell with clear molecular genetic background, the pharmacological action molecular mechanism research in animals can be carried out.
Owner:KUNMING UNIV OF SCI & TECH
Preparation method of Rag1 gene defect animal model and application thereof
ActiveCN110564773ANo leaksGuaranteed success rateHydrolasesStable introduction of DNAImmunodeficiencyWilms' tumor
The invention provides a preparation method of a Rag1 gene defect animal model, which utilizes a gene editing technology to destroy a Rag1 gene coding region of a mouse, detects the model through immune system index evaluation and pathological histological examination, and verifies that the obtained mouse model is successfully constructed. The immunodeficiency model is helpful for research in thefields of tumor transplantation, immunology, inflammation and the like.
Owner:GEMPHARMATECH CO LTD
Human angioma rat animal model and its configuration method
The present invention transplants hemangioma endothelial cell and tumor cell or tumor cell releasing product isolating cultured from human hemangioma structure, to immunologic deficiency mouse hypodermic, to obtain human hemangioma mouse animal model. The present invented technology method is portably in technical operation and with high model success ratio, the experimental evidence proving that said blood vessel endothelial cell in hemangioma model established is human hemangioma endothelial cell, and said model having the pathology property of human angiomatous intumescentia blood sinus and rebirth capillary vessel. Said hemangioma animal model can be used in system researching angiomatous pathogenesis screening human angiomatous therapeutic drug.
Owner:SUZHOU BOJUHUA BIOMEDICAL TECH CO LTD
Artificially designed HIV (human immunodeficiency virus)-infection-resisting polypeptide, composition and application
InactiveCN103122024APeptide/protein ingredientsAntiviralsAcquired immunodeficiencyIMMUNOLOGIC DEFICIENCY
The invention belongs to the field of biological medicine, relates to artificially designed HIV (human immunodeficiency virus)-infection-resisting polypeptide, in particular to polypeptide as shown in a formula I of Xa1AAXd1Xe1BB-Xa2AAXd2Xe2BB-Xa3AAXd3Xe3BB-Xa4AAXd4Xe4BB-Xa5AAXd5Xe5BB (formal I), and a derivative, a stereoisomer or physiological-toxicity-free salt thereof. The invention further relates to a pharmaceutical composition containing the polypeptide in the formula I and the derivative, the stereoisomer or the physiological-toxicity-free salt of the polypeptide, and application of the polypeptide in the formula I and the derivative, the stereoisomer or the physiological-toxicity-free salt of the polypeptide to treatment or prevention of related diseases and particular acquired immunodeficiency syndrome (AID) caused by HIV infection.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Regulators of NFAT
Disclosed are methods of identifying an agent that modulates an NFAT regulator protein. One such method comprises contacting at least one test agent with a recombinant cell comprising at least one NFAT regulator protein or fragment or derivative thereof, assessing the effect of the test agent on an activity, interaction, expression, or binding to the NFAT regulator protein or fragment or derivative thereof, and identifying the test agent that has an effect on an activity, interaction, expression, or binding to the NFAT regulator protein or fragment or derivative thereof, whereby the identified test agent is characterized as an agent that modulates an NFAT regulator protein. Methods of identifying an agent that modulates intracellular calcium, methods to screen for an agent that modulates NFAT regulator function, methods to diagnose unexplained immunodeficiency in a subject, and methods for identifying an agent for treating or preventing a disease or disorder associated with a NFAT regulator protein or calcium signaling are also disclosed.
Owner:CHILDRENS MEDICAL CENT CORP
Lipsotrichia-stopping hair-growing preparation
InactiveCN1640381AImprove applicabilityNo hair lossPeptide/protein ingredientsUnknown materialsVitamin E AcetateAlopecia seborrhoeica
The present invention discloses an alopecia-stopping hair-growing preparation which can be used for curing alopecia resulted from various causes, specially for effectively curing alopecia seborrhoeica, immunologic deficiency alopecia and alopecia due to chemotherapy. It is made up by using thymic peptide, nettle extract, ginkgo leaf extract, vitamin E acetate, preservative, isopropanol, ethyl alcohol and water according to a certain mixing ratio. Said invention also provides its preparation method.
Owner:胡敏灿
Thymosin alpha 1 active segment cyclicpeptide analogue and its poly glycol derivative
The present invention relates to a kind of cyclopeptide derivative containing natural or artificial amino acid substituted active thymosin alpha-1 segment, and the preparation process thereof, medicine composition and their medicines for treating or preventing diseases related to immune deficiency, hypoimmunity, etc.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
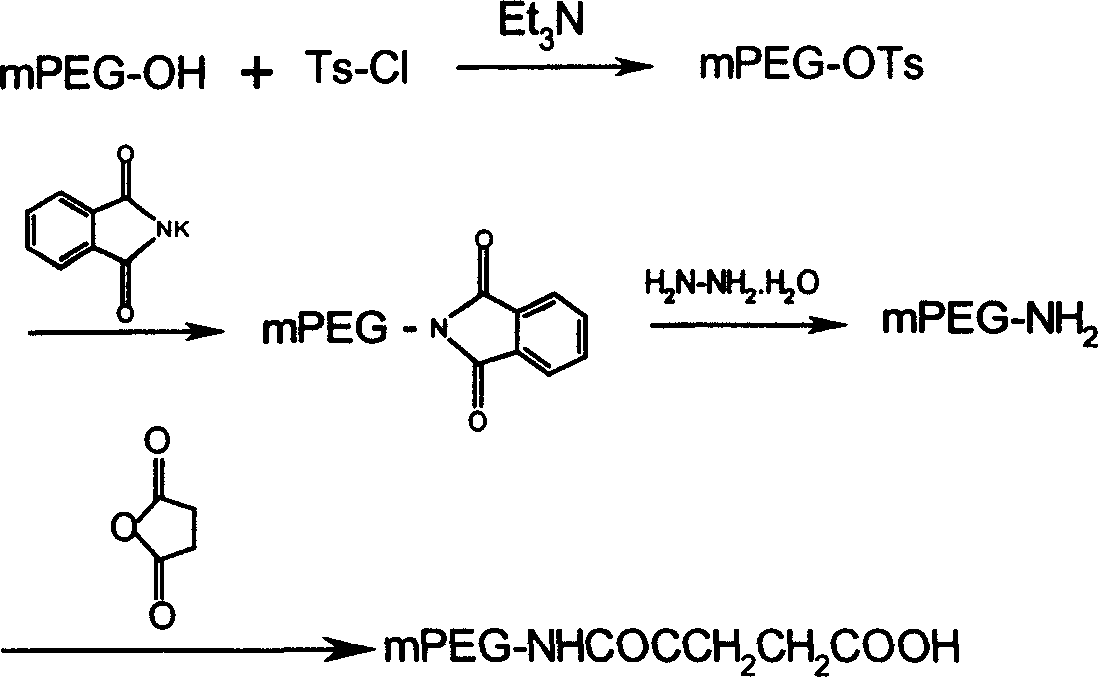
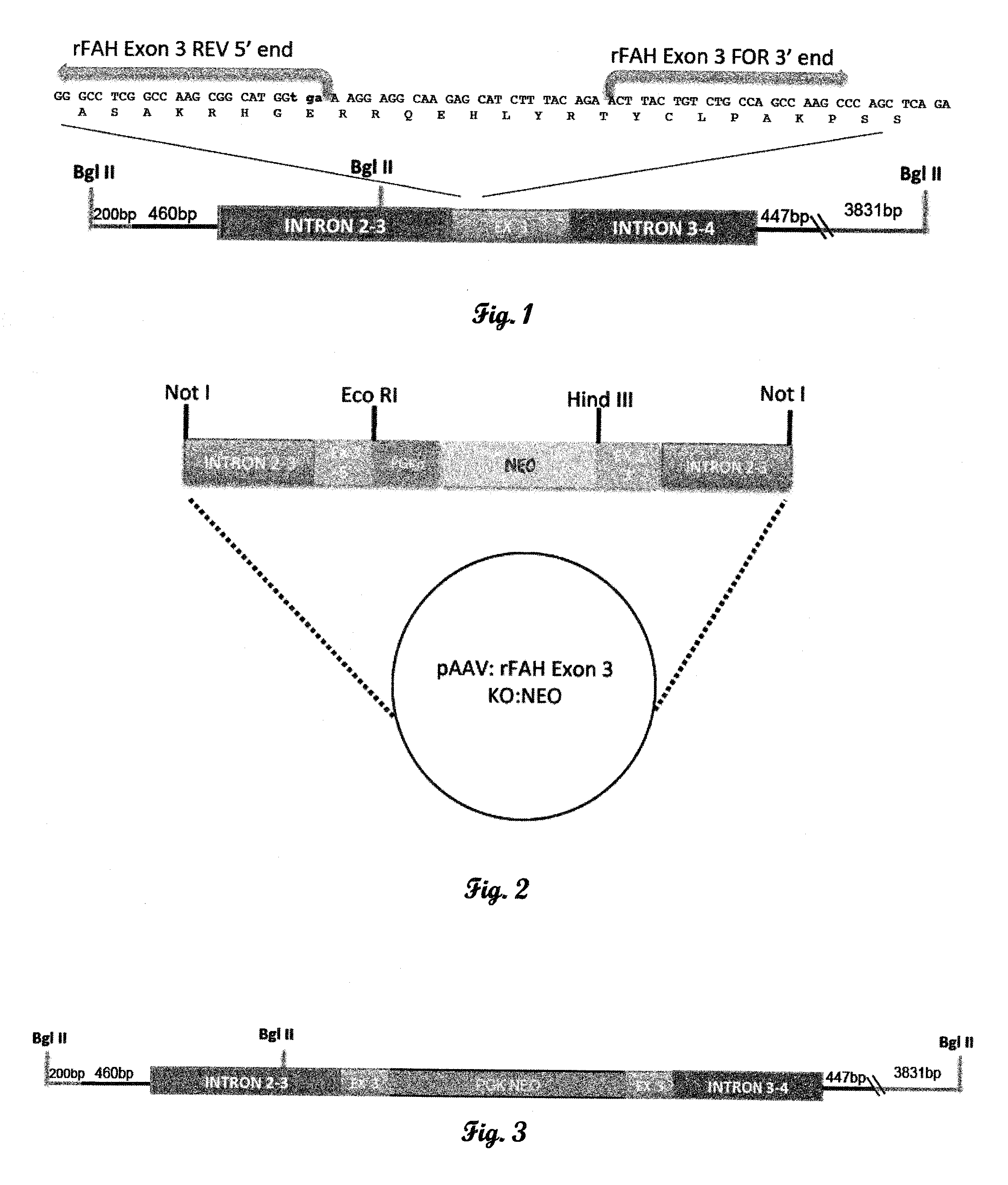
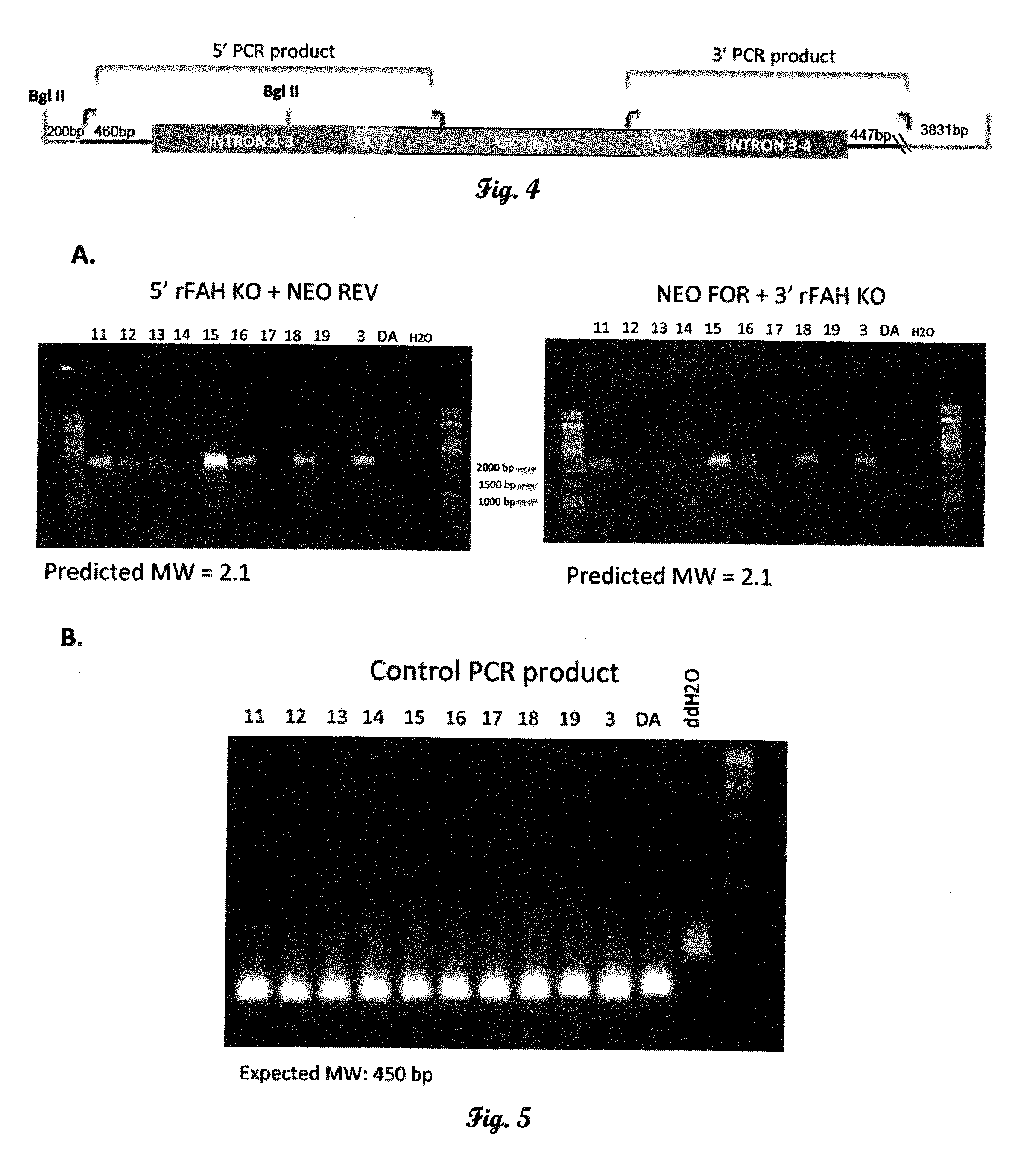
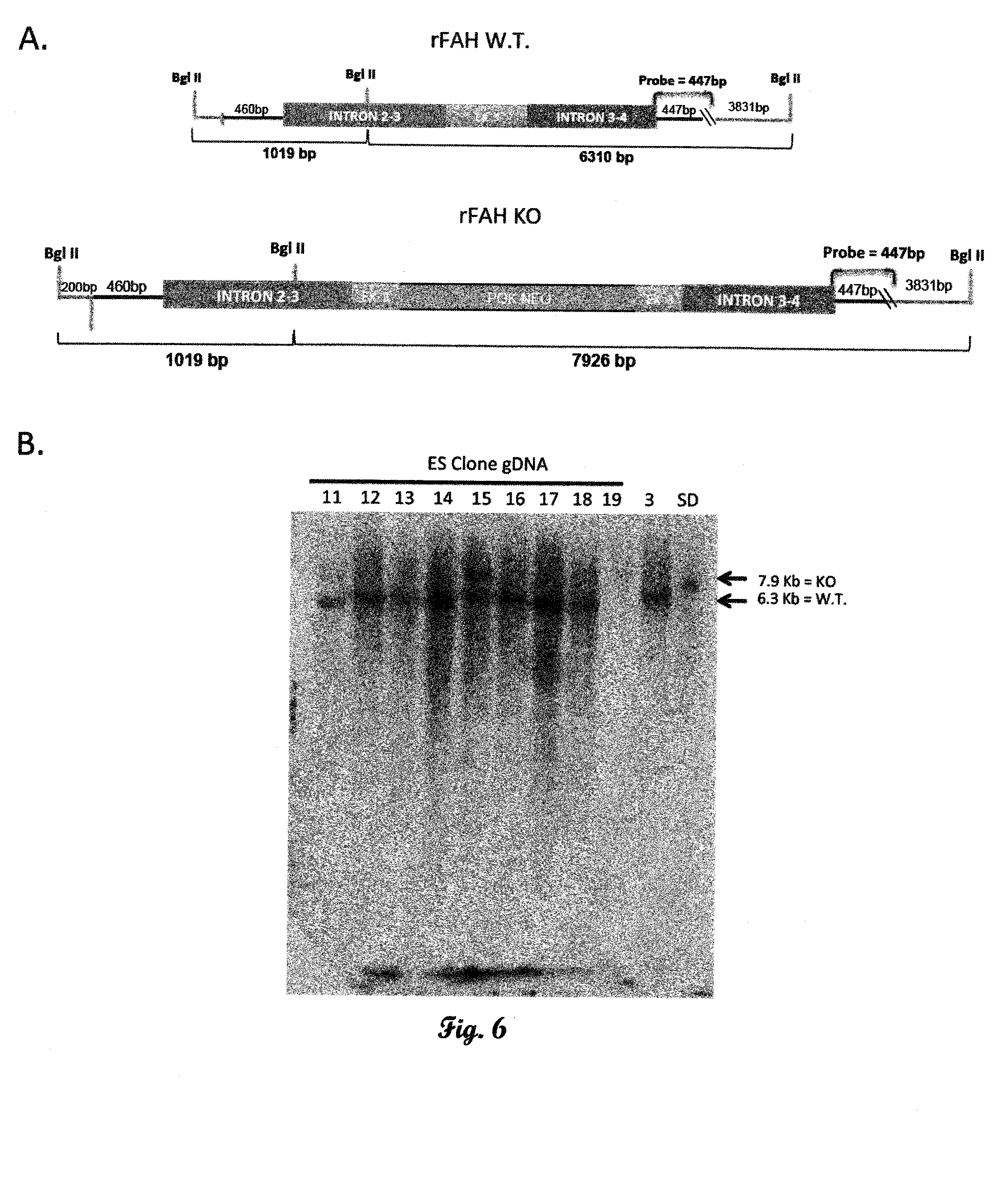
Fumarylacetoacetate hydrolase (FAH)-deficient and immunodeficient rats and uses thereof
Described herein are rats with a hepatic deficiency comprising decreased function, activity, or expression of an enzyme in the tyrosine catabolic pathway (such as fumarylacetoacetate hydrolase), and methods of using the same for in vivo engraftment and expansion of heterologous hepatocytes, such as human hepatocytes, analysis of human liver disease, and analysis of xenobiotics. Also disclosed is the use of immunodeficient rats for the engraftment and expansion of heterologous hepatocytes.
Owner:YECURIS CORP
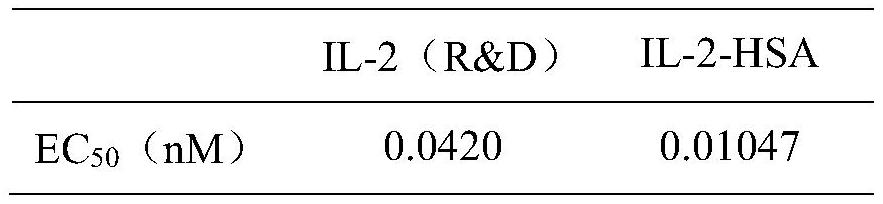
Long-acting recombinant human interleukin 2 fusion protein and preparation method and application thereof
InactiveCN113789346AProlonged plasma half-lifeHigh expressionPeptide/protein ingredientsAntipyreticDiseaseWhite blood cell
The invention discloses a fusion protein of interleukin 2 and human serum albumin. Plasmids with a fusion gene of interleukin 2 and human serum albumin are electrically transferred into CHO cells to obtain a CHO monoclonal cell strain capable of stably and efficiently expressing human recombinant protein. The monoclonal cell strain of the invention can secrete and express the fusion protein of the interleukin 2 and the human serum albumin, and the fusion protein can prolong the plasma half-life period of the human interleukin 2 and can be used for preparing human interleukin 2 drugs or drugs for treating various diseases, such as tumors and immunodeficiency diseases.
Owner:BEIJING VDJBIO
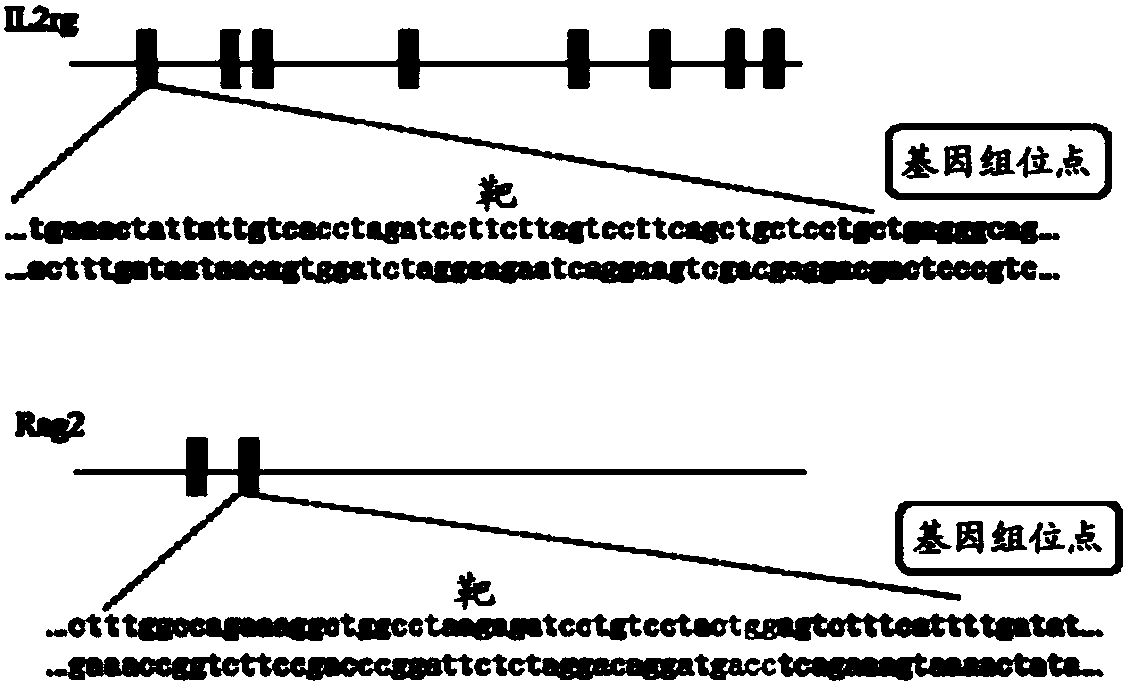
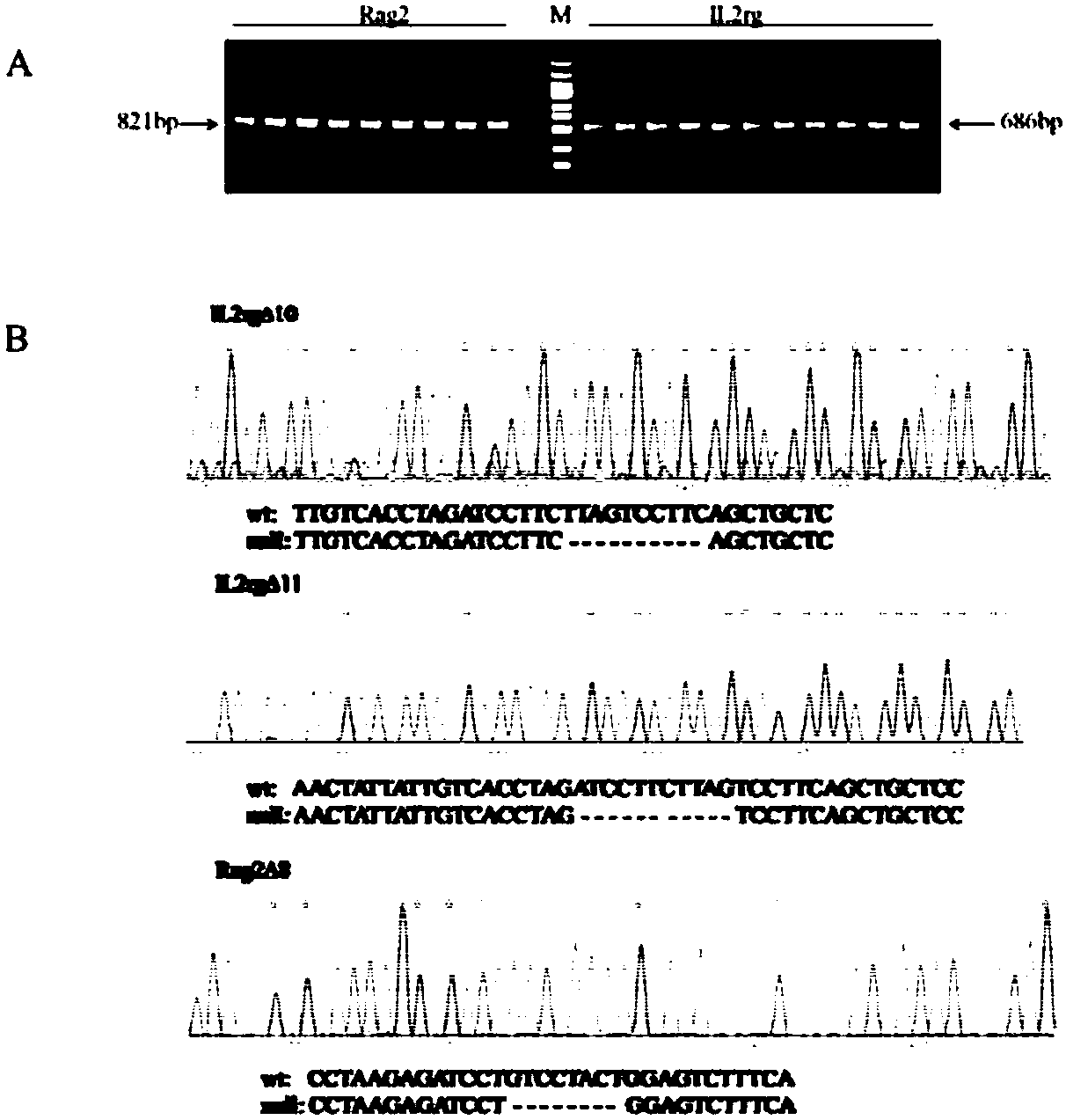
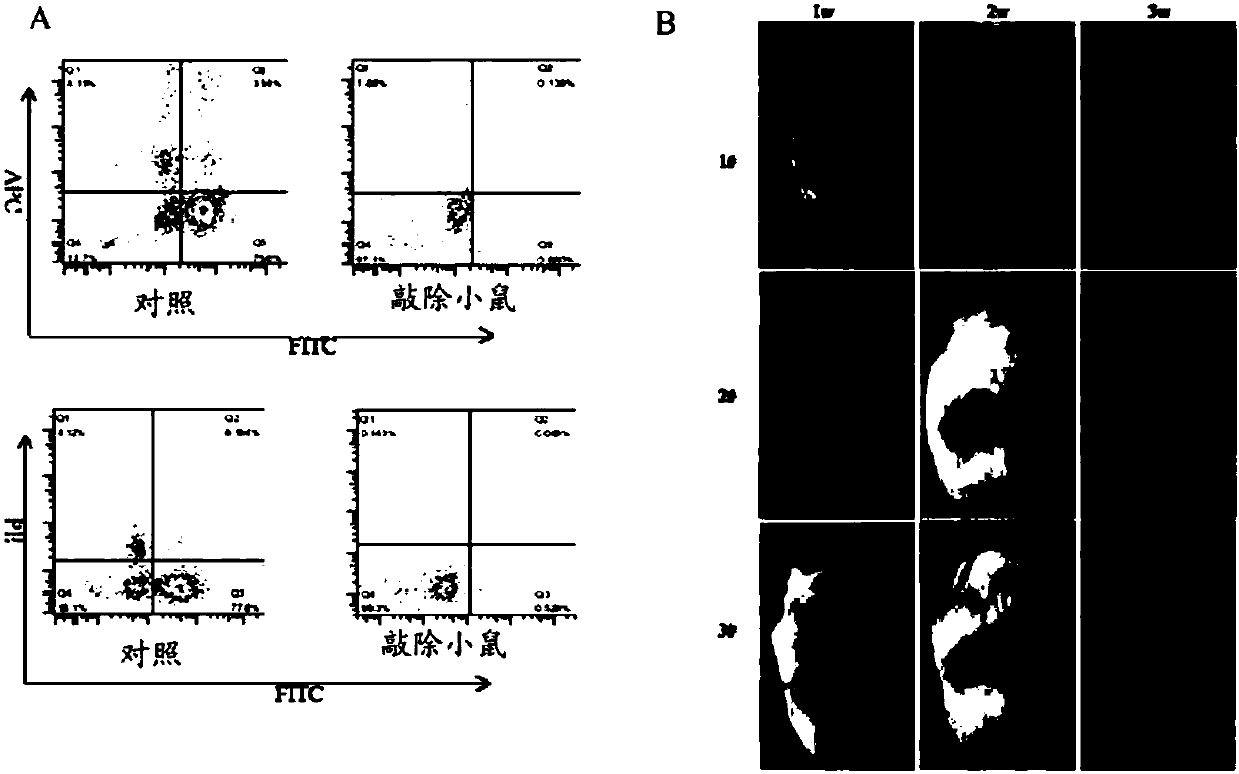
Construction and application of severe combined immunodeficiency animal model
InactiveCN107760720AReliable knockout efficiencyTumor cells growing wellHydrolasesMicroinjection basedIL2RG geneSevere Combined Immunodeficiency Syndrome
The invention relates to construction and related application of a severe combined immunodeficiency animal model. According to the construction method, Rag2 genes for encoding animal T and B cells andIl2rg genes involved in encoding cytokine receptors are removed in a targeted mode by means of a CRISPR / Cas9 technology, and the construction of the animal model with a higher immunodeficiency degreeis achieved.
Owner:北京艾德摩生物技术有限公司 +1
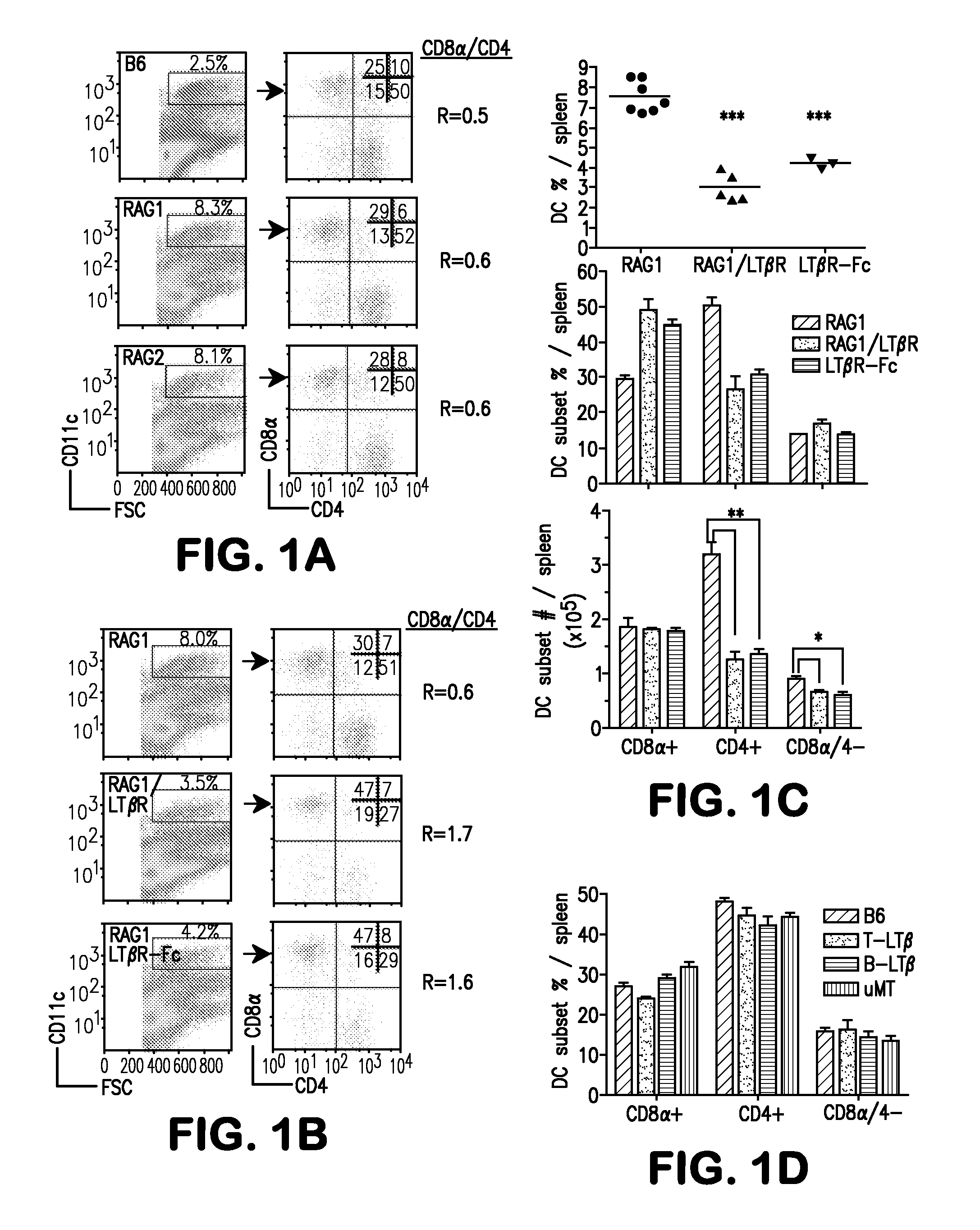
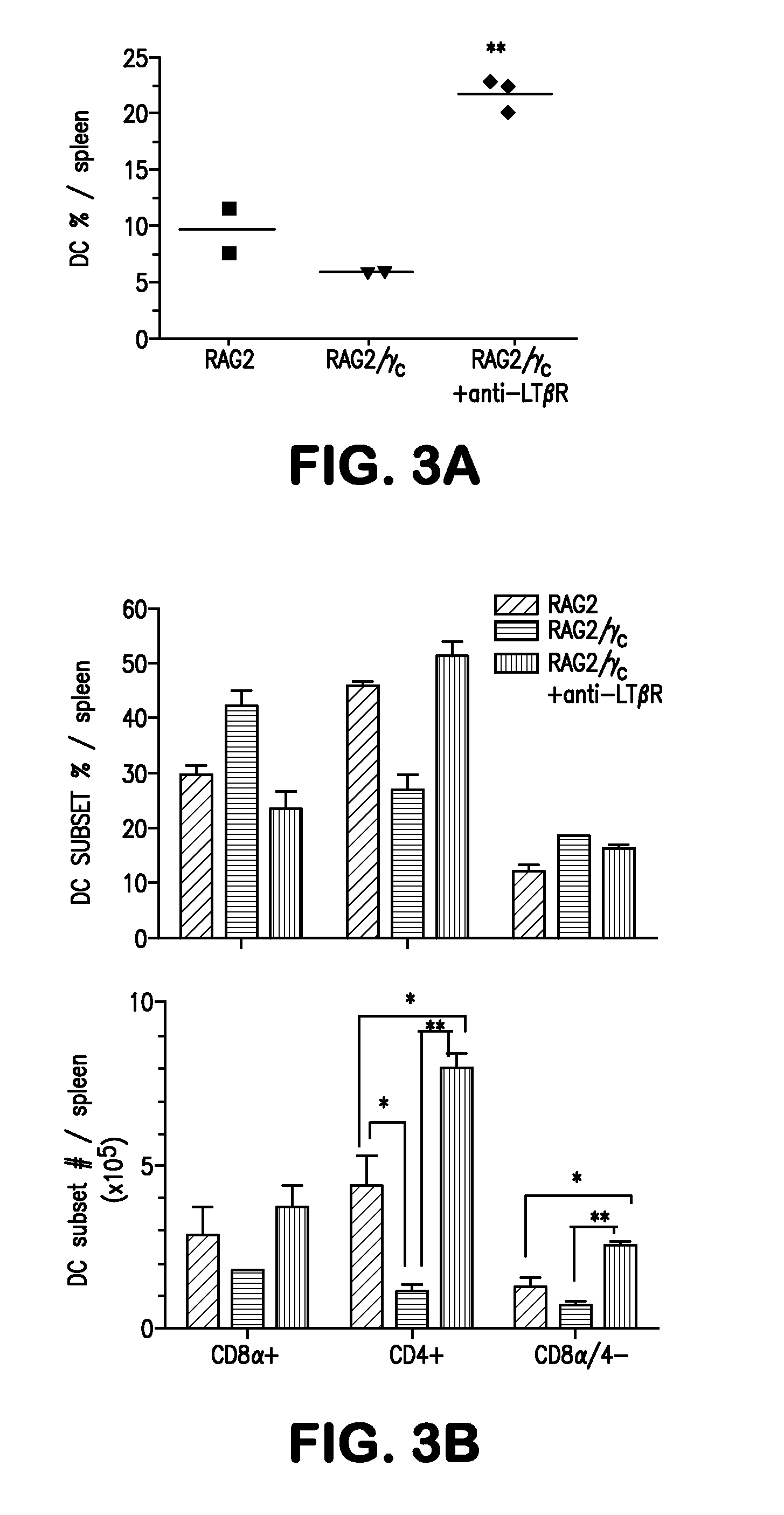
Method for restoring dendritic cell populations
ActiveUS8153123B2Restore and increase DC populationReduced DC populationMicrobiological testing/measurementAntibody ingredientsDendritic cellAgonist
The present invention provides methods for restoring and increasing dendritic cell populations in a subject by modulation of the lymphotoxin-β receptor (LTβR) via LTβR agonists. The invention also provides methods for screening for agents capable of restoring or increasing dendritic cell populations. The invention further provides a method for the treatment of immunodeficiency by administration of an LTβR agonist.
Owner:LA JOLLA INST FOR ALLERGY & IMMUNOLOGY
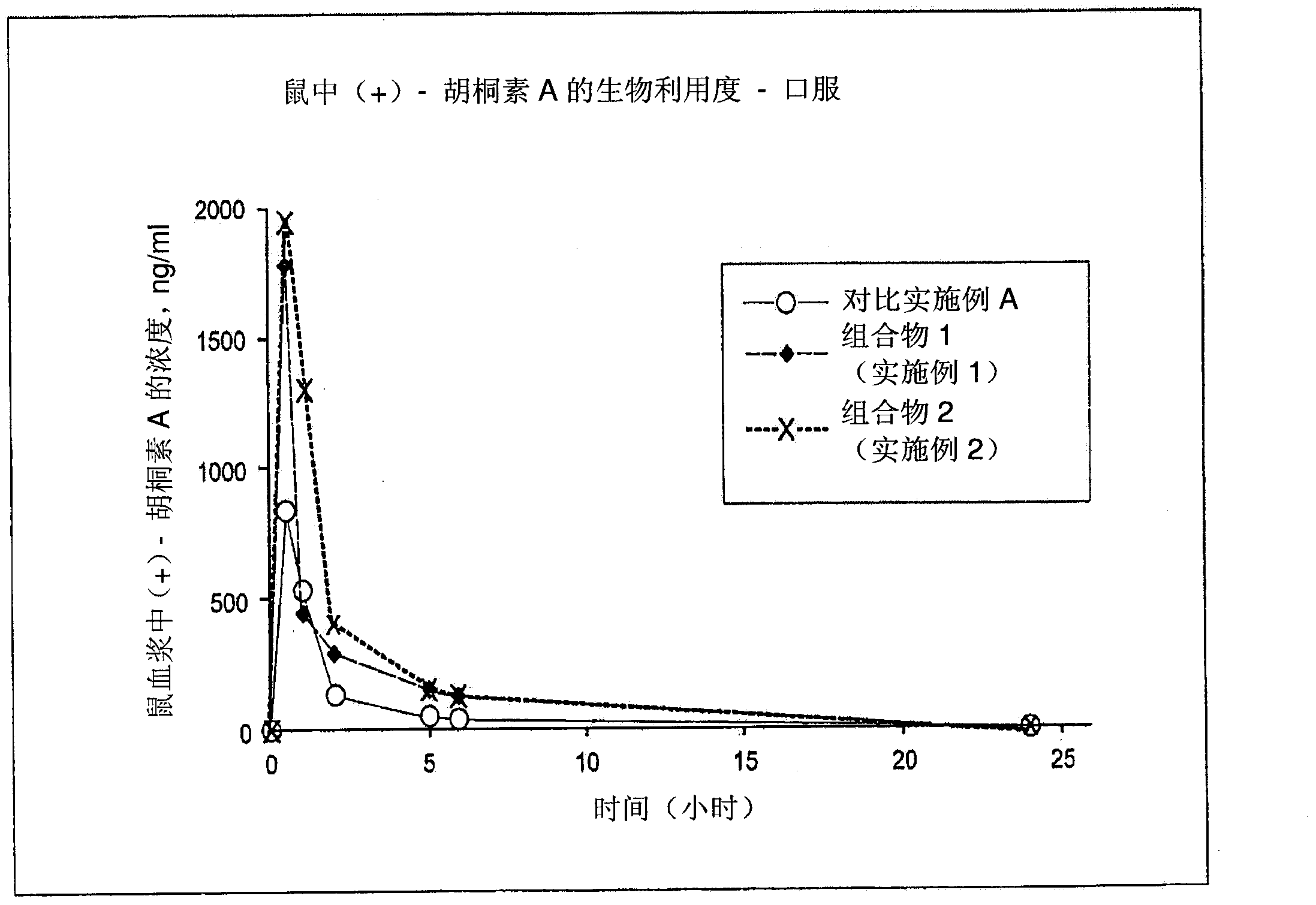
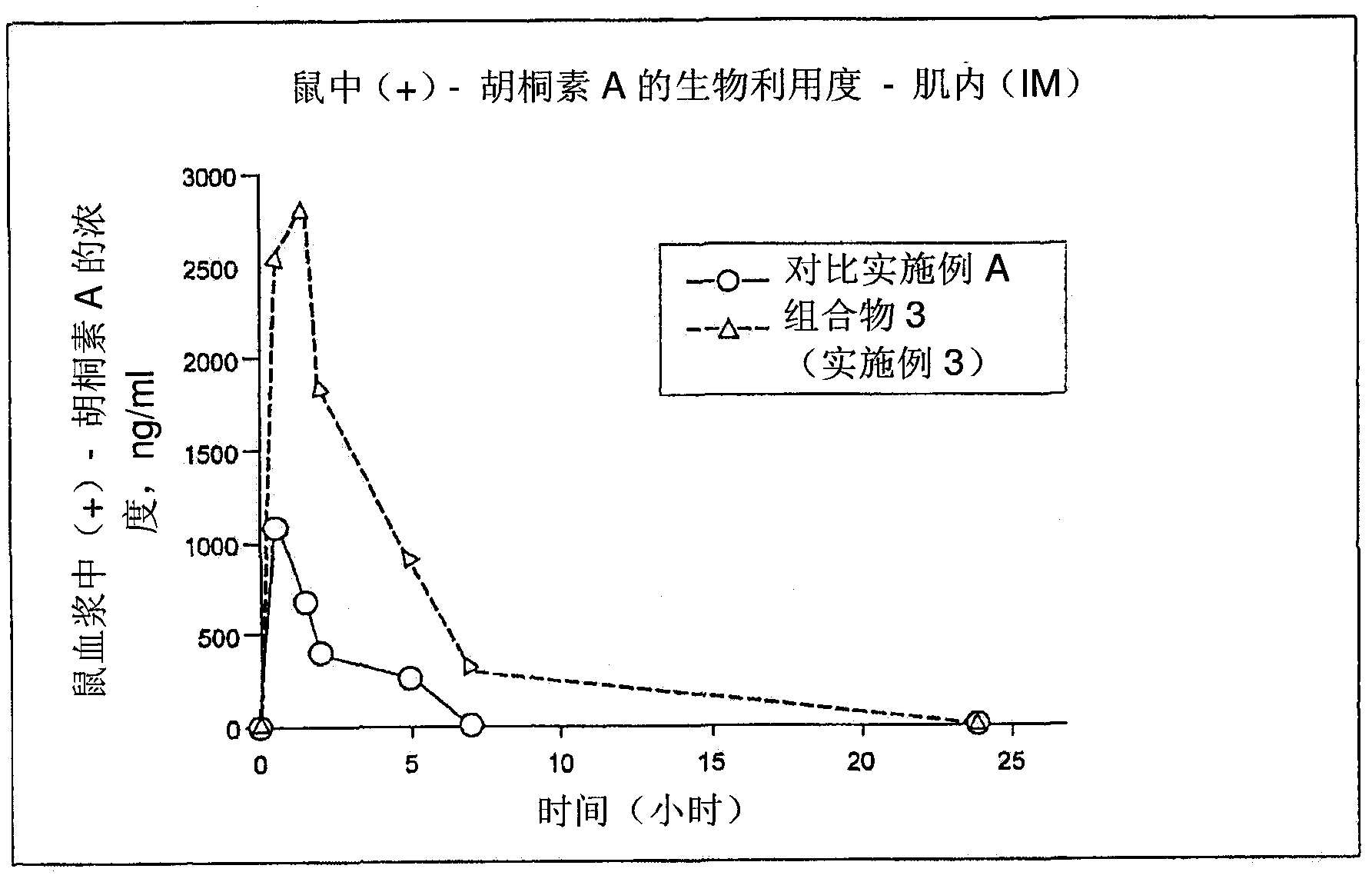
Pharmaceutical compositions for calanolides, their derivatives and analogues, and process for producing the same
The present invention relates to pharmaceutical compositions of calanolides, their derivatives and analogues, and process for producing the same having enhanced solubility and bioavailability for oral or parenteral administration. The invention further provides for a method of using the disclosed compositions for the treatment and prevention of retroviral diseases such as human immunodeficiency, specifically HTV-1 and mycobacterial diseases especially tuberculosis infections in mammals, particularly humans.
Owner:CRAUN RES
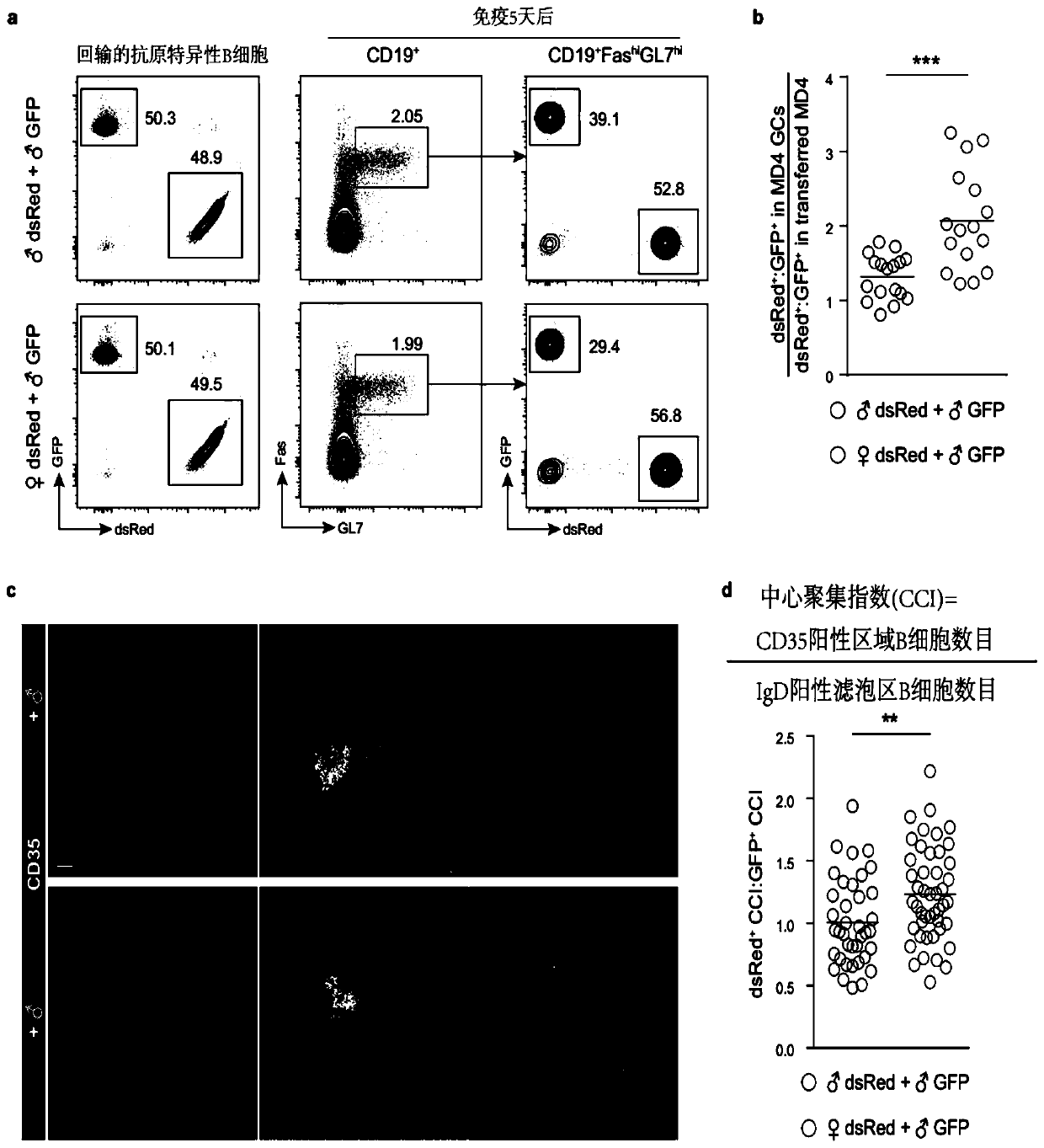
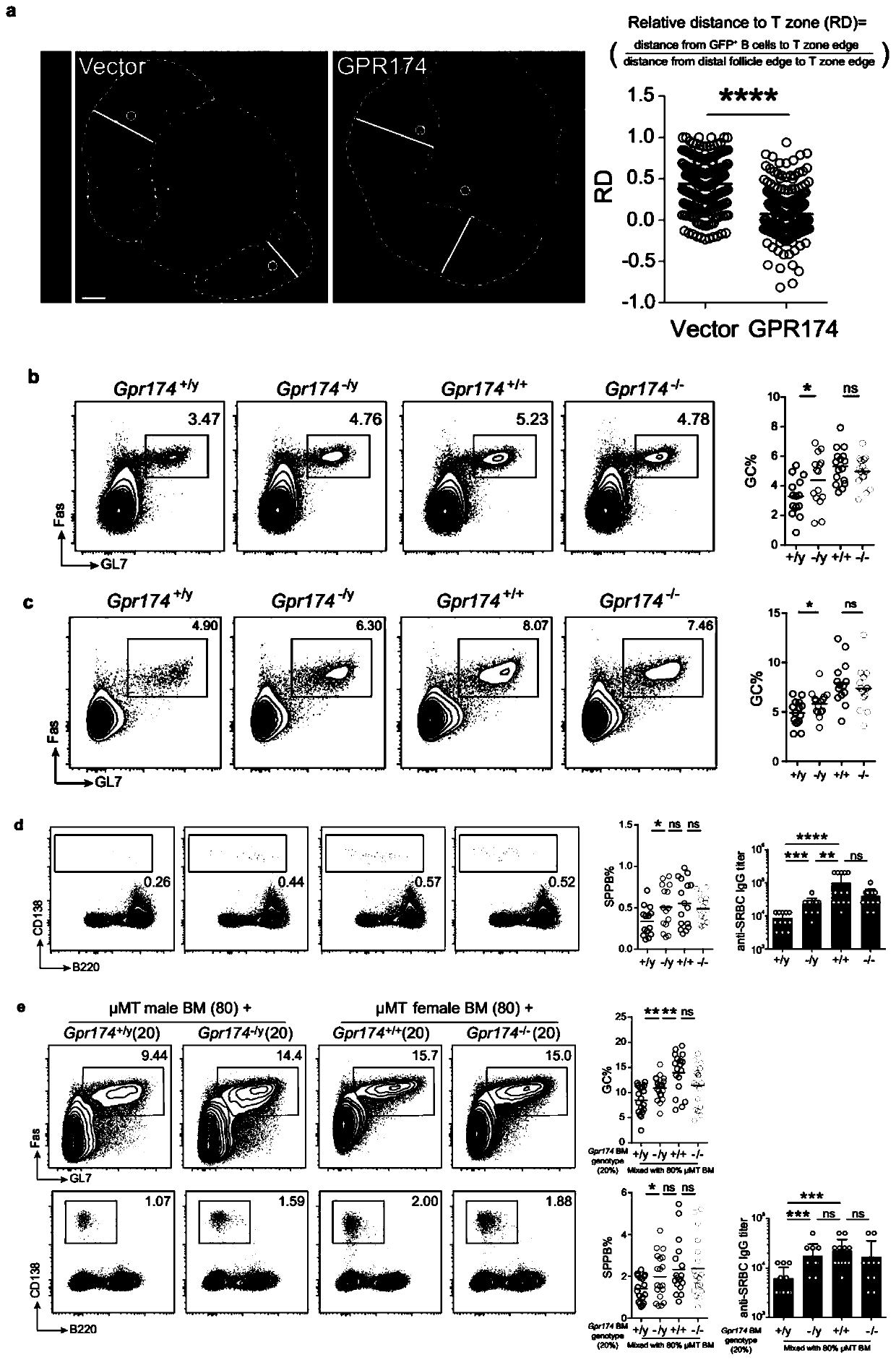
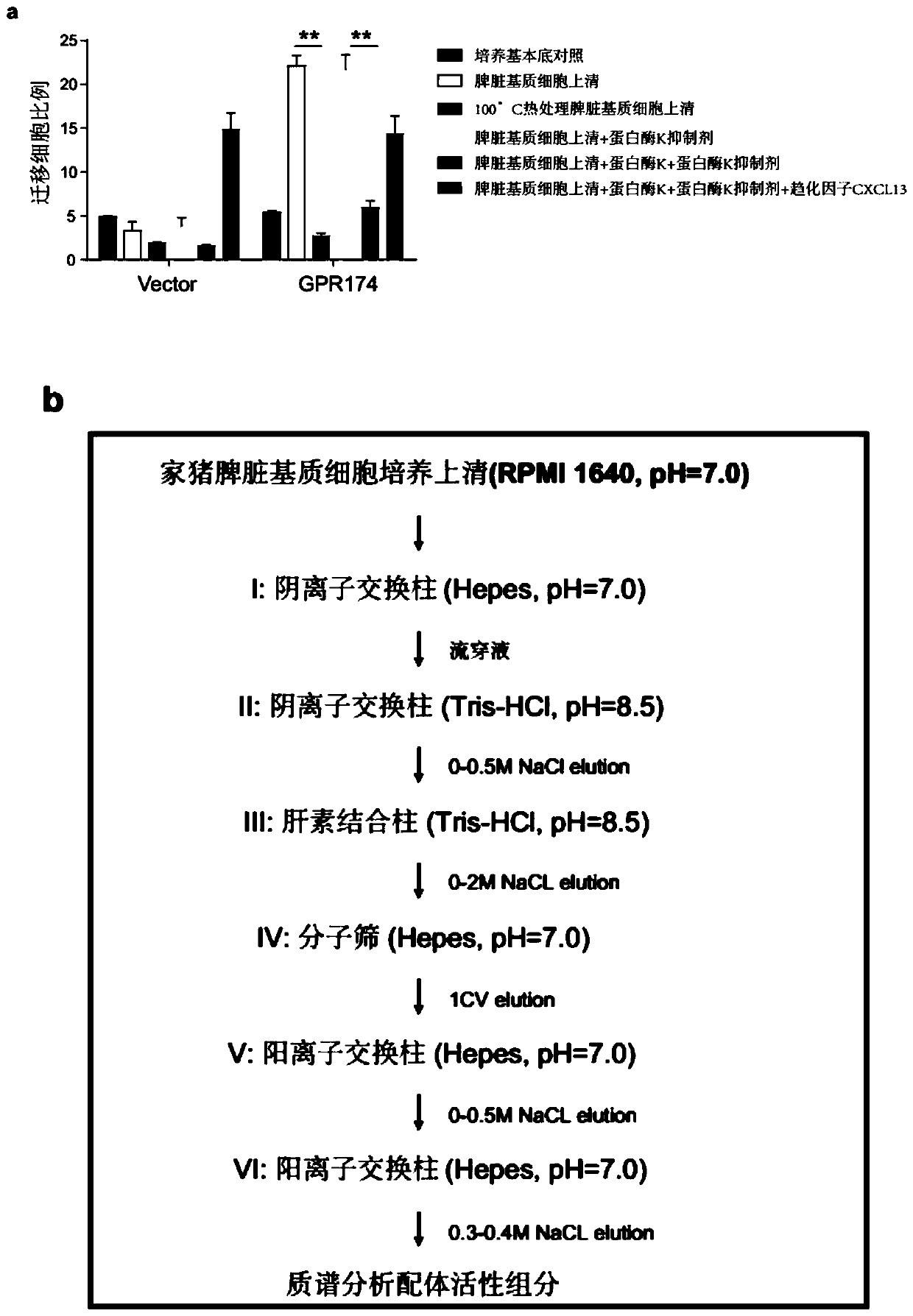
Immunology functions and purpose of GPR174
ActiveCN110804642APrecise screeningMicrobiological testing/measurementImmunological disordersPharmaceutical drugIMMUNOLOGIC DEFICIENCY
The invention provides immunology functions and purpose of GPR174. The GPR174 comprises GPR174 and / or CCL21 and / or CCL19 inhibition type antibodies or antagonists or expression inhibitors or low-expression cell medicines, can be effectively used for increasing humoral immunity response of organisms particularly male organisms or treating or preventing immune deficiency, so that the level of a specific antibody produced in accordance with external antigens of an inoculator can be increased, or the level of a specific antibody produced in accordance with tumor relevant antigens of a tumor patient can be increased, and tumor immunity treatment effects can be improved.
Owner:TSINGHUA UNIV
System for preparing severe immunodeficiency swine-derived recombinant cells with joint knockout of four RRIP genes
ActiveCN112522257ACloning efficiency is lowIncrease the cost of feedingHydrolasesAntibody mimetics/scaffoldsRAG2Medicine
The invention discloses a system for preparing severe immunodeficiency swine-derived recombinant cells with joint knockout of four RRIP genes, and particularly relates to a system for preparing severeimmunodeficiency swine-derived recombinant cells with joint knockout of four genes such as an RAG1 gene, an RAG2 gene, an IL2RG gene and a PRKDC gene. The invention provides an sgRNA (Ribonucleic Acid) combination. The sgRNA combination consists of sgRNARAG1-g4, sgRNARAG2-g2, sgRNAIL2RG-g7 and sgRNAAPRKDC-g6. The target sequence binding region of the sgRNARAG1-g4 is shown as nucleotides at the first to the twentieth site in SEQ ID NO: 9; the target sequence binding region of the sgRNARAG2-g2 is shown as nucleotides at the first to the twentieth site in SEQ ID NO: 13; the target sequence binding region of the sgRNAIL2RG-g7 is shown as nucleotides at the first to the twentieth site in SEQ ID NO: 24; and the target sequence binding region of the sgRNAPRKDC-g6 is shown as nucleotides at the first to the twentieth site in the SEQ ID NO: 34. The invention lays a solid foundation for preparation of a severe immunodeficiency swine model, and has important application values for research and development of severe immunodeficiency medicine.
Owner:NANJING KGENE GENETIC ENG CO LTD
Recombinant cell with IL2RG gene and ADA gene knocked out jointly and application of recombinant cell in preparation of immunodeficient swine model
PendingCN112522258ACloning efficiency is lowReduce feeding costsHydrolasesAntibody mimetics/scaffoldsNucleotideIMMUNOLOGIC DEFICIENCY
The invention discloses a recombinant cell with IL2RG gene and ADA gene knocked out jointly and application of the recombinant cell in preparation of an immunodeficient swine model. The invention provides application of an sgRNA (small guide ribonucleic acid) combination consisting of sgRNAIL2RG-g7 and sgRNAADA-g7 in preparation of a kit. The invention further provides an sgRNA (small guide ribonucleic acid) combination. The sgRNA combination is composed of sgRNAIL2RG-g7 and sgRNAADA-g7. The target sequence binding region of the sgRNAIL2RG-g7 is shown as nucleotides at the first to the twentieth site in SEQ ID NO: 12; and the target sequence binding region of the sgRNAADA-g7 is shown as nucleotides at the first to the twentieth site in SEQ ID NO: 22. The invention also discloses application of the sgRNA combination in preparation of recombinant cells and application of the sgRNA combination in preparation of an immunodeficient animal model. The invention lays a solid foundation for preparation of a severe immunodeficiency swine model, and has important application values for research and development of severe immunodeficiency medicine.
Owner:NANJING KGENE GENETIC ENG CO LTD
Highly metastatic model of human melanoma, cell subline, creation methods, and dynamic detection of metastasis
The invention discloses a highly metastatic model of human melanoma, a highly metastatic cell subline of the human melanoma, creation methods for the highly metastatic model and the highly metastatic cell subline, and the dynamic detection of metastasis. The subcutaneously-transplanted mouse highly metastatic model and the corresponding cell subline are established in an in-vivo screening way in a mouse with severe combined immune deficiency (SCID) by using mouse lung metastasis, namely human malignant melanoma cell strain A375 pulmonary metastasis, wherein the highly metastatic cell subline of the human melanoma is A375sci, and has a human tumor cell karyotype; and 60 to 75 hypo-triploid-dominated chromosomes are acrocentric and have a heteroploid karyotype. The cell subline has the two routes of metastasis of blood trails and lymph. The in-vivo screening of the highly-metastatic model is performed by using animals with severe immune deficiency, and is expressed and applied in nude mice. A method for detecting Alu genes by using a polymerase chain reaction (PCR) method is simple, highly sensitive and highly specific, and can be used for detecting organ metastasis, particularly micrometastasis, in a human tumor animal-xenotransplantation model.
Owner:SHANGHAI INST OF ONCOLOGY
Mouse-derived oral squamous cell carcinoma cell strain, and preparation method and application thereof
PendingCN113201495ASolve the problem of poor tumor formation effectCompound screeningCell dissociation methodsCarcinoma cell lineSquamous Carcinomas
The invention discloses a mouse-derived oral squamous cell carcinoma cell strain,and a preparation method and application thereof, and the preparation method comprises the following steps: firstly, adding 4-nitroquinoline-N-oxide into mouse drinking water, inducing mouse oral squamous cell carcinoma, and obtaining mouse oral squamous cell carcinoma tumor tissues; carrying out digestion and primary culture on the mouse tumor tissue to obtain mouse tumor primary cells; selecting a monoclonal cell from the primary cells by adopting a monoclonal screening method; and purifying the monoclonal cells, carrying out continuous passage, and screening out a cell strain to obtain the mouse oral squamous cell carcinoma cell strain. The mouse squamous cell carcinoma cells can form tumors on both immunodeficient nude mice and immune-healthy C57BL / 6J mice, so that the problem that the existing human oral squamous cell carcinoma cell line has poor tumor forming effect on immune-healthy mice is solved; the invention has an important value in establishment of a tumor immune mouse model and research of a subsequent interaction mechanism of a tumor and an immune system.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
HIV antibody immune magnetic beads rapid detection reagent kit
ActiveCN101266247BIncreased sensitivitySensitivity up toMaterial analysisDiseaseImmunodeficiency virus
The invention relates to a quick detection kit for detecting human immunologic deficiency disease (HIV) using nm immunomagnetic beads, related to biology applied technique field. The detected sample is added on the sampling pad of the test paper from the sampling hole and the sample dissolves the mark beads and chromatography is performed on the NC membrane, and then the data on the test paper isdetected using magnetic force detector in preset time and the result is decided based on the read data. The kit is used for screening or clinical diagnosis of the HIV infection with high sensitivity,specificity, quickspeed, convenience and suitable for detection on site and real time recording and storing the result.
Owner:天津中新科炬生物制药股份有限公司
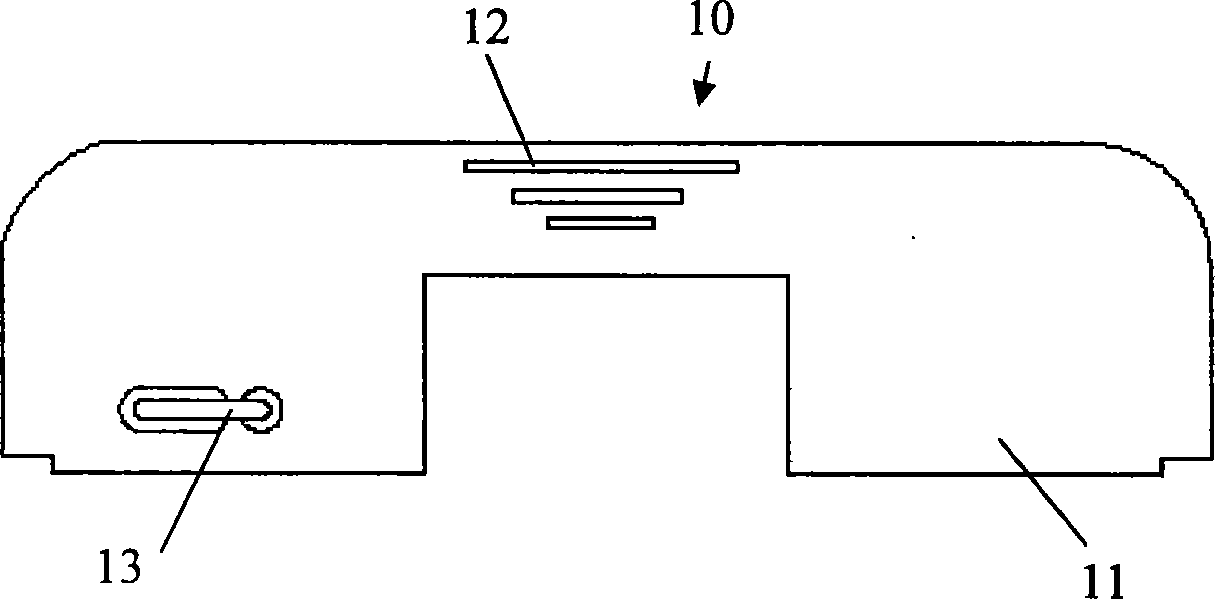
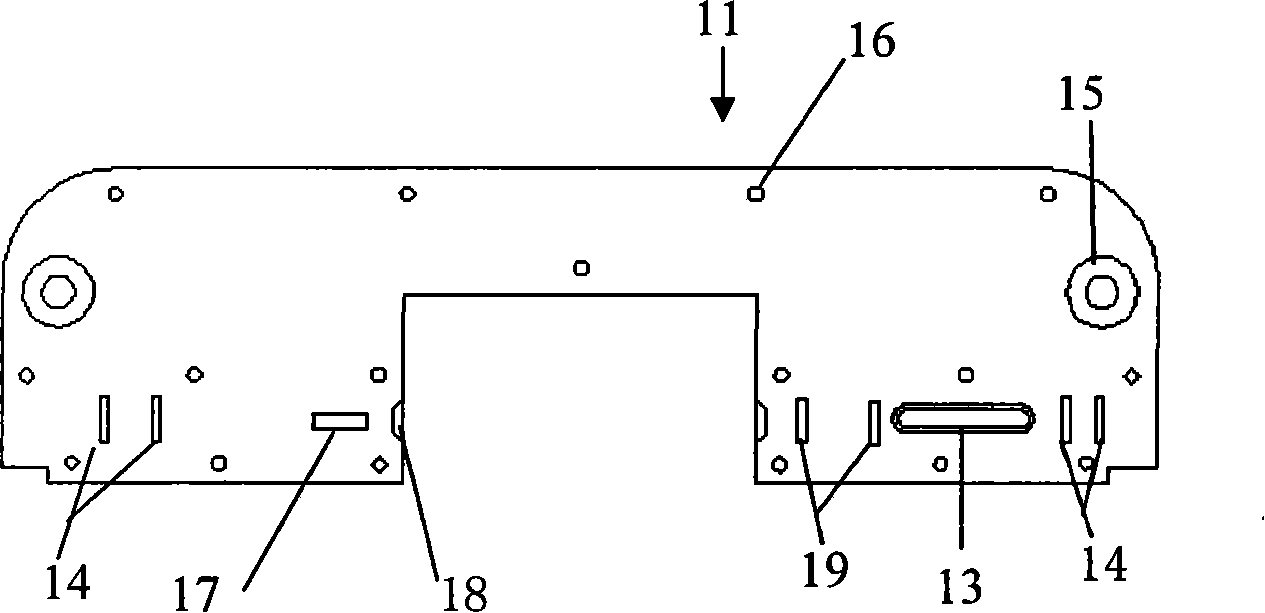
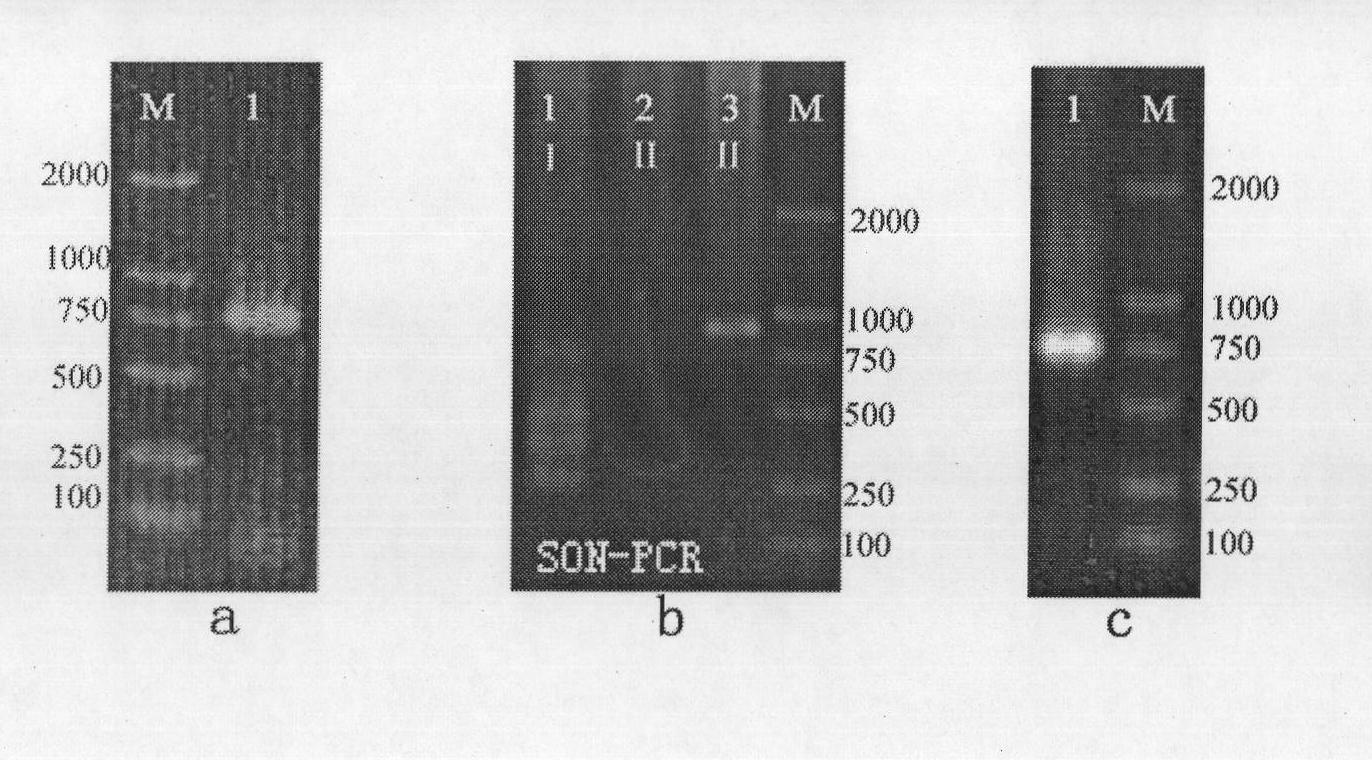
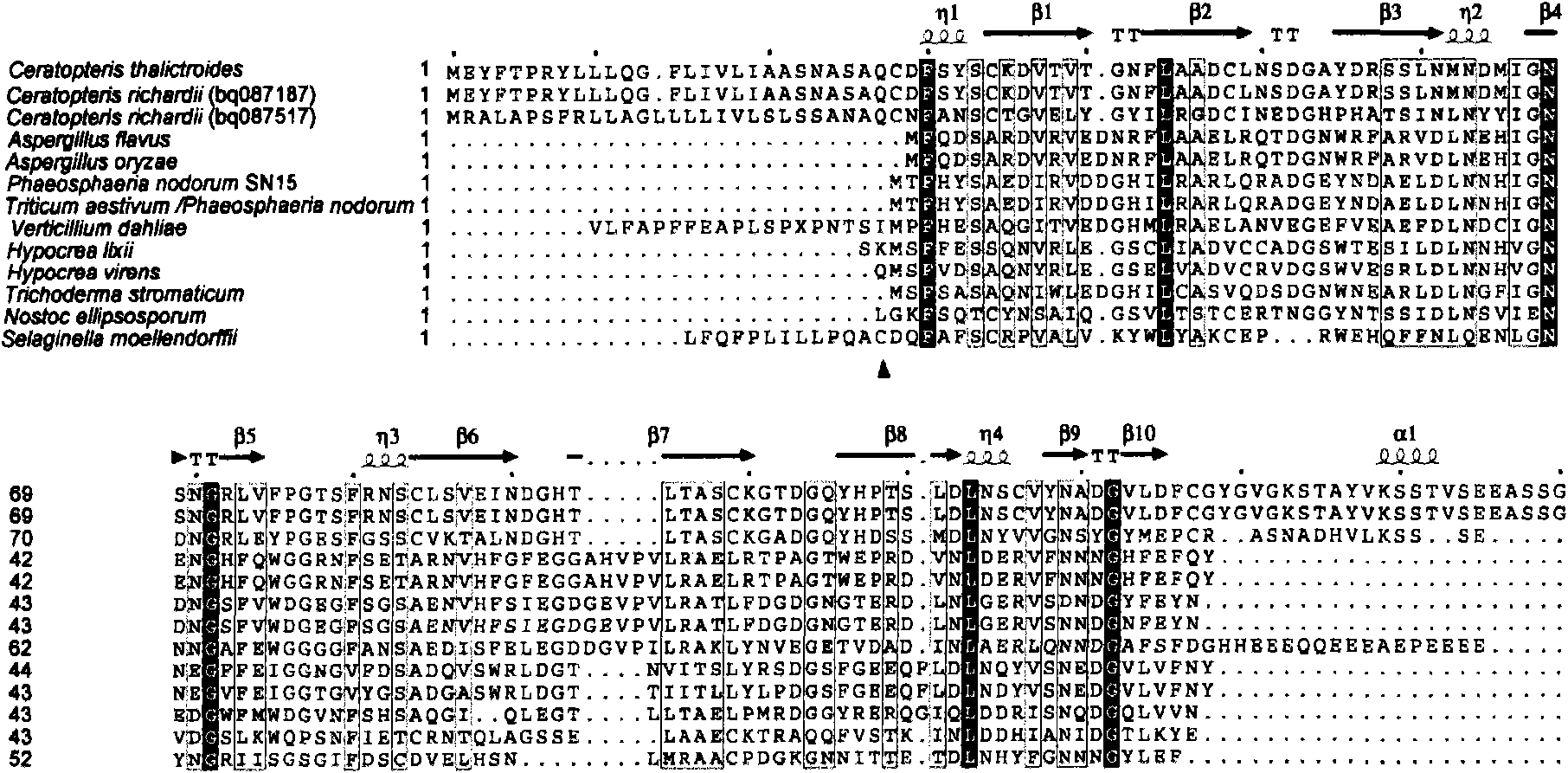
Ceratopteris pteridoides gene for resisting human immunologic deficiency disease toxoprotein congeners, and preparation method and application thereof
InactiveCN101838654AScreening Technology ImprovementsConvenient researchPeptide/protein ingredientsAntiviralsCeratopteris pteridoidesDisease
The invention discloses a ceratopteris pteridoides gene for resisting human immunologic deficiency disease toxoprotein congeners, and a preparation method and application thereof. The preparation method comprises the following steps: 1, extracting total DNA by using an improved CTAB method, and preserving; 2, amplifying the intermediate segment of the ceratopteris pteridoides gene for resisting human immunologic deficiency disease toxoprotein congeners; 3, amplifying 5' flanking sequence of the ceratopteris pteridoides gene for resisting human immunologic deficiency disease toxoprotein congeners; 4, amplifying 3' flanking sequence of the ceratopteris pteridoides gene for resisting human immunologic deficiency disease toxoprotein congeners; and 5, recycling and cloning the PCR product: recycling the intermediate segment of the target gene, and 5' and3' flanking sequence segments, transforming, culturing and selecting white colonies; and 6, judging the clone, measuring the sequence and splicing. The gene can be used for regulation and control on expression of potential antivirus genes in plant research, carrying out research into plant antivirus gene engineering, and further establishes a ceratopteris pteridoides CVNH protein preparation system, thereby finally realizing large-scale industrial production.
Owner:WUHAN BOTANICAL GARDEN CHINESE ACAD OF SCI
Novel immunodeficient rat for modeling human cancer
InactiveUS20210092942A1Receptors for cytokines/lymphoines/interferonsAnimals/human peptidesHuman cancerAssay
Disclosed herein are methods and compositions for performing assays for determining efficacy of drugs using rat SCID models that exhibit excellent take rates, and excellent tumor growth rates. The methods and compositions offer dramatically improved efficiencies compared to corresponding mouse equivalents.
Owner:HERA TESTING LAB INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com