Fumarylacetoacetate hydrolase (FAH)-deficient and immunodeficient rats and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of the AAV-rFAH:PGK-NEO Null Targeting Vector and Production of FAH KO Rat ES Cells
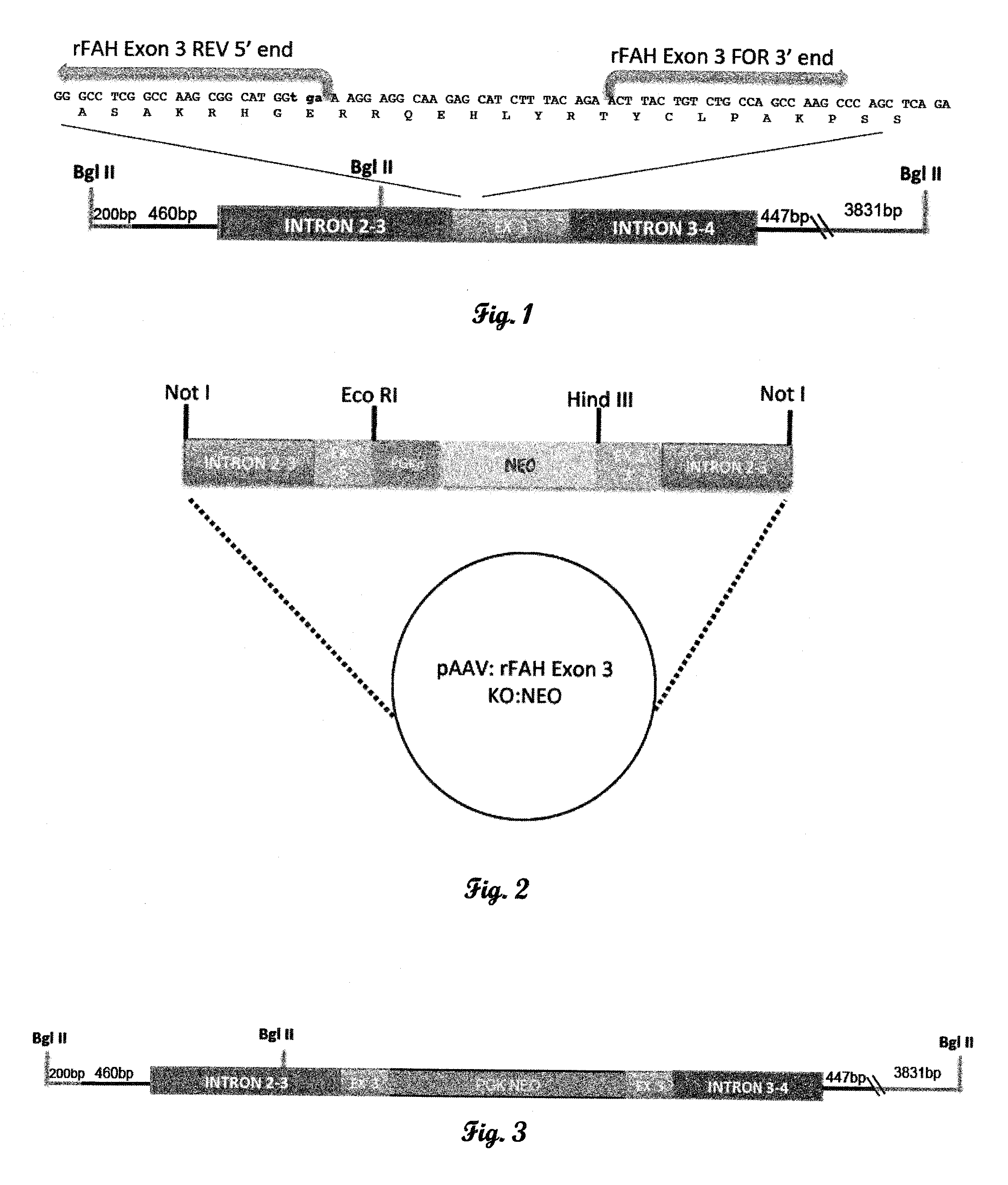
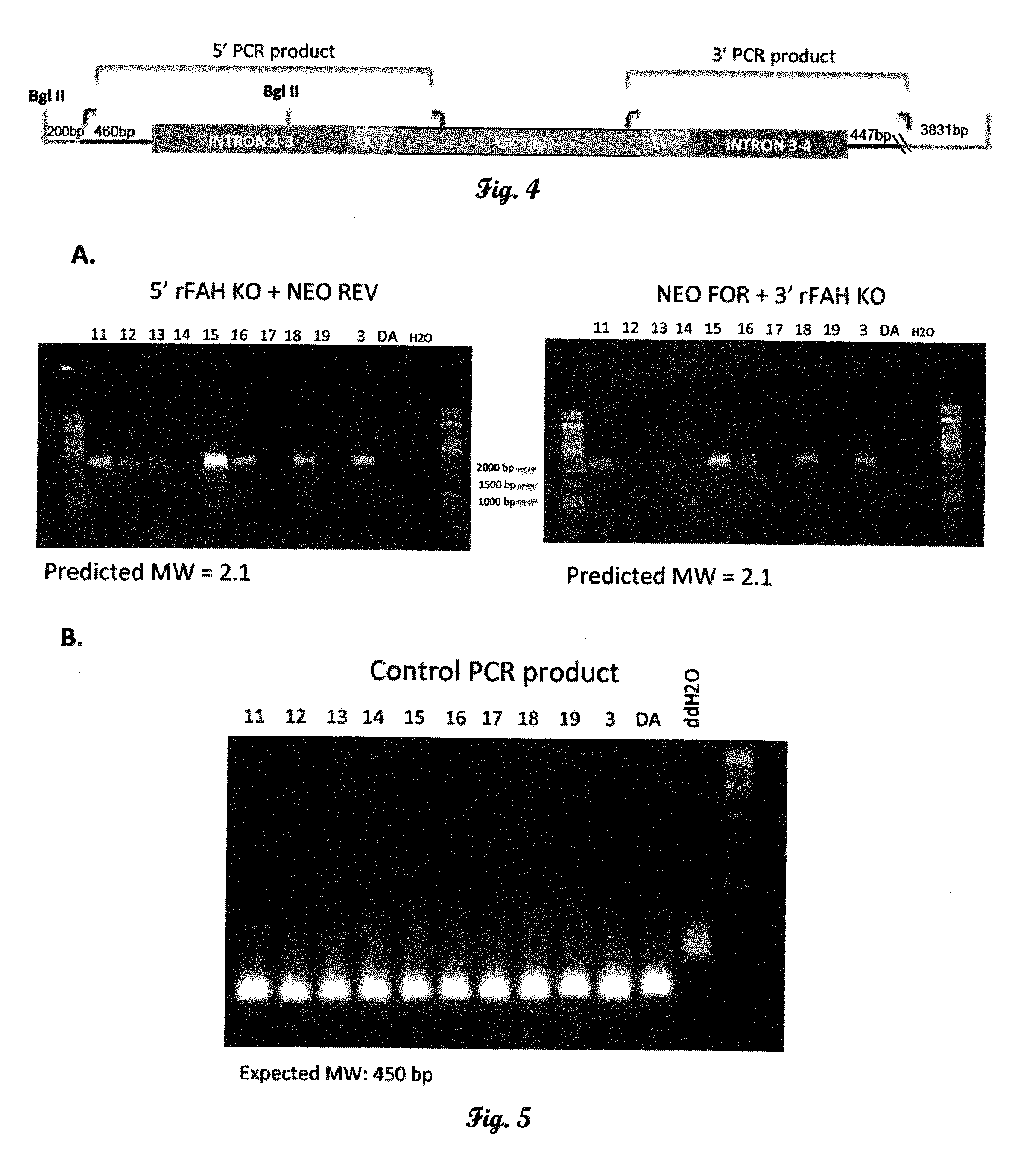
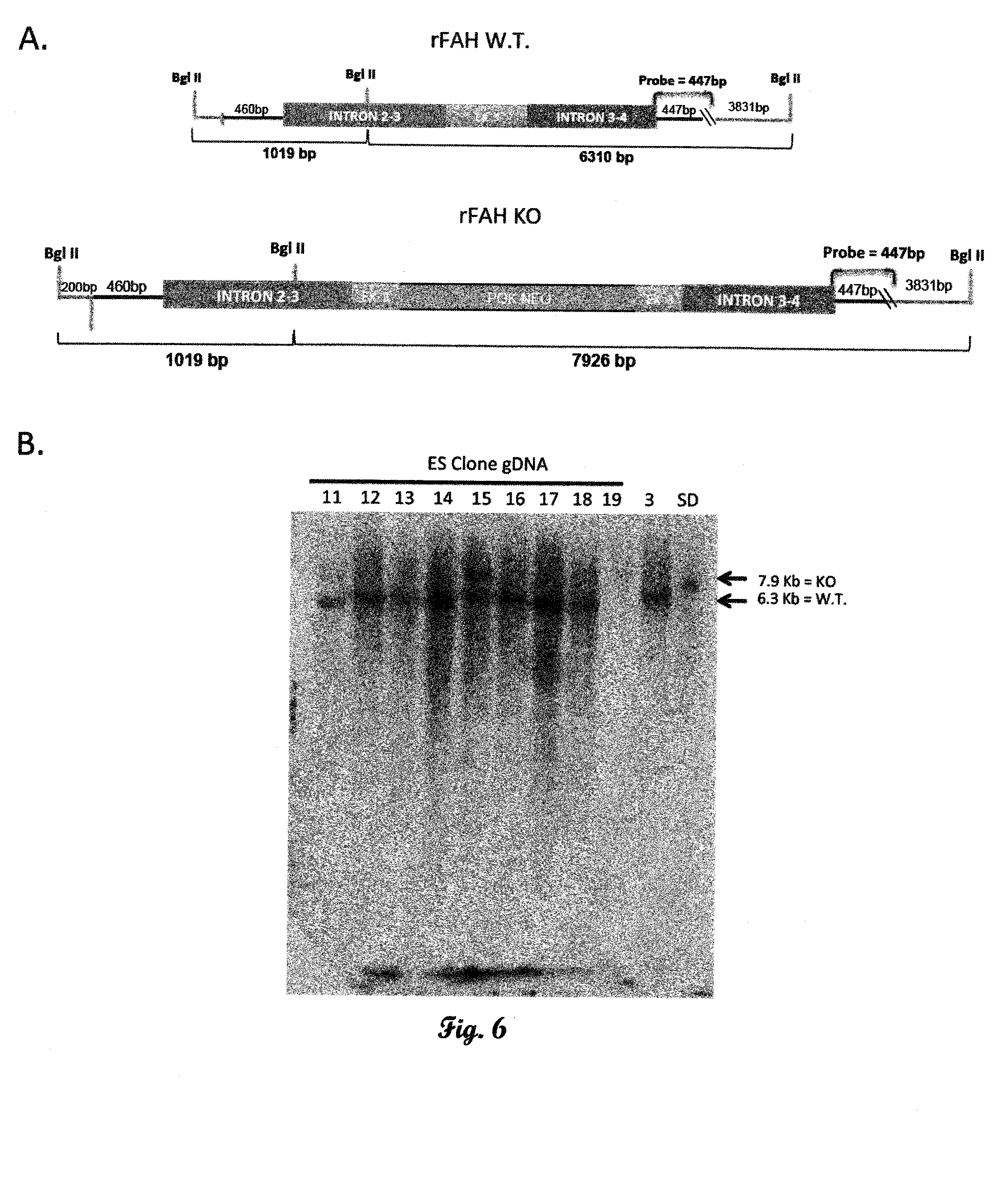
[0177]The rat FAH KO construct targets exon 3 of the FAH gene. Rat ES cell genomic DNA was used to generate 5′ and 3′ FAH homology regions for the targeting construct. A 5′ FAH homology region was generated using a forward primer 1.5 kb upstream of exon 3 (intron 1-2; Table 1), and the reverse primer containing a stop codon ending 54 bp into exon 3 of the FAH gene (SEQ ID NO:1; FIG. 1). A 3′ FAH homology region was generated using a forward primer starting 90 bp into exon3 and ending in intron 4-5 (1.5 kb; Table 1). The two 1.5 kb PCR products were cloned, sequenced and ligated to a PGK:NEO selection cassette totaling 4.7 kb (FIG. 2). The nucleotide sequence of the targeting cassette is set forth herein as SEQ ID NO:2.
[0178]The 4.7 kb fragment was ligated into the pcDNA 3.1 vector, which was linearized and transfected into rat 208D fibroblast cells to examine NEO resistance. The cells were ...
example 2
Generation of FAH KO Rats and Transplantation of Human Hepatocytes
[0182]FAH KO rats were generated by injecting FAH KO ES cells into rat blastocysts, which are then transferred to E3.5 pseudo-pregnant SD rats. The chimeric progeny from these rats are bred to generate heterozygous FAH KO rats by germline transmission. Male and female heterozygotes are then further bred to generate FAH knockout rats. The deletion of the FAH gene allows for the induction of liver damage to aid in high levels of engraftment and repopulation of the liver with transplanted human hepatocytes.
[0183]Prior to transplantation with human hepatocytes, acute liver damage is induced in the rats by dosing with a recombinant adenovirus expressing the plasminogen-like urokinase enzyme. Twenty-four hours later the human hepatocytes are delivered via intra-splenic injection where the hepatocytes will travel through the vasculature to reach the liver. In addition, immune suppression drugs are given to the rats before, d...
example 3
Generation of the Il2rg Construct
[0184]The rat Il2rg construct targets exon 3 of the Il2rg gene. Rat ES cell genomic DNA was used to generate 5′ and 3′ Il2rg homology regions for the targeting construct. A 5′ Il2rg homology region was generated using a forward primer 1.46 kb upstream of exon 3 (Table 2), and a reverse primer containing a stop codon ending 40 bp inside exon 3 of the IL2rg gene. A 3′ Il2rg homology region was generated using a forward primer containing 40 bp of exon3 and ending in exon 6 (1.5 kb; Table 2). The two 1.5 kb PCR products were cloned, sequenced and ligated to a PGK:NEO selection cassette totaling 4.7 kb, similar to that of the FAH construct described in Example 1. The construct was sequenced and used for viral production.
TABLE 2Primers used in the design of the rIL2rg constructPrimer Sequence SEQPrimer Location5′ to 3′ID NO:5′ Rat IL2rg FORgtagcgaattcgcggccgctg17attggattctcggtgtga5′ Rat IL2rg REVggatagaattcgtcagtggct18gcactcctggaatgtattatt3′ Rat IL2rg FORg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com