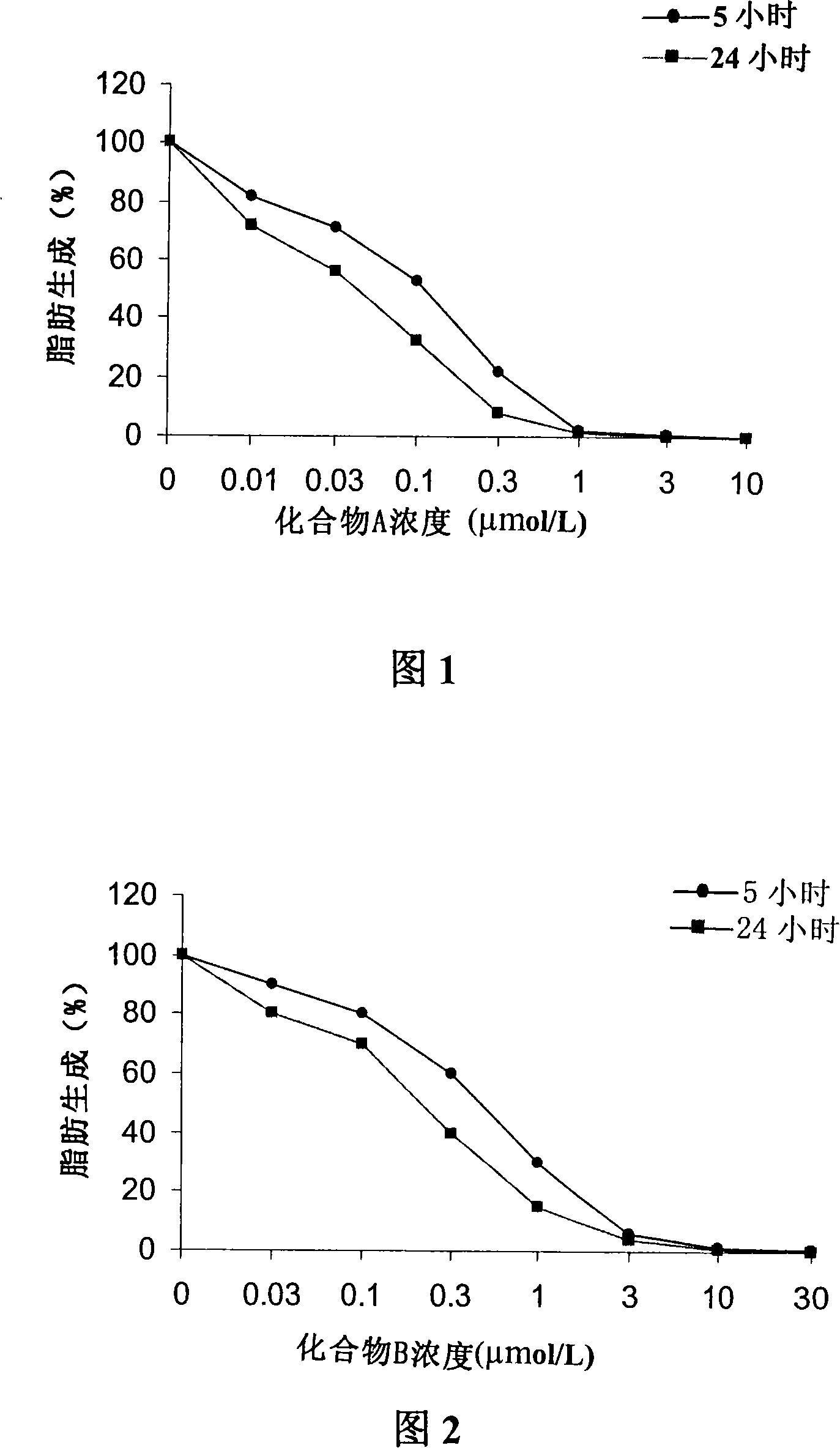
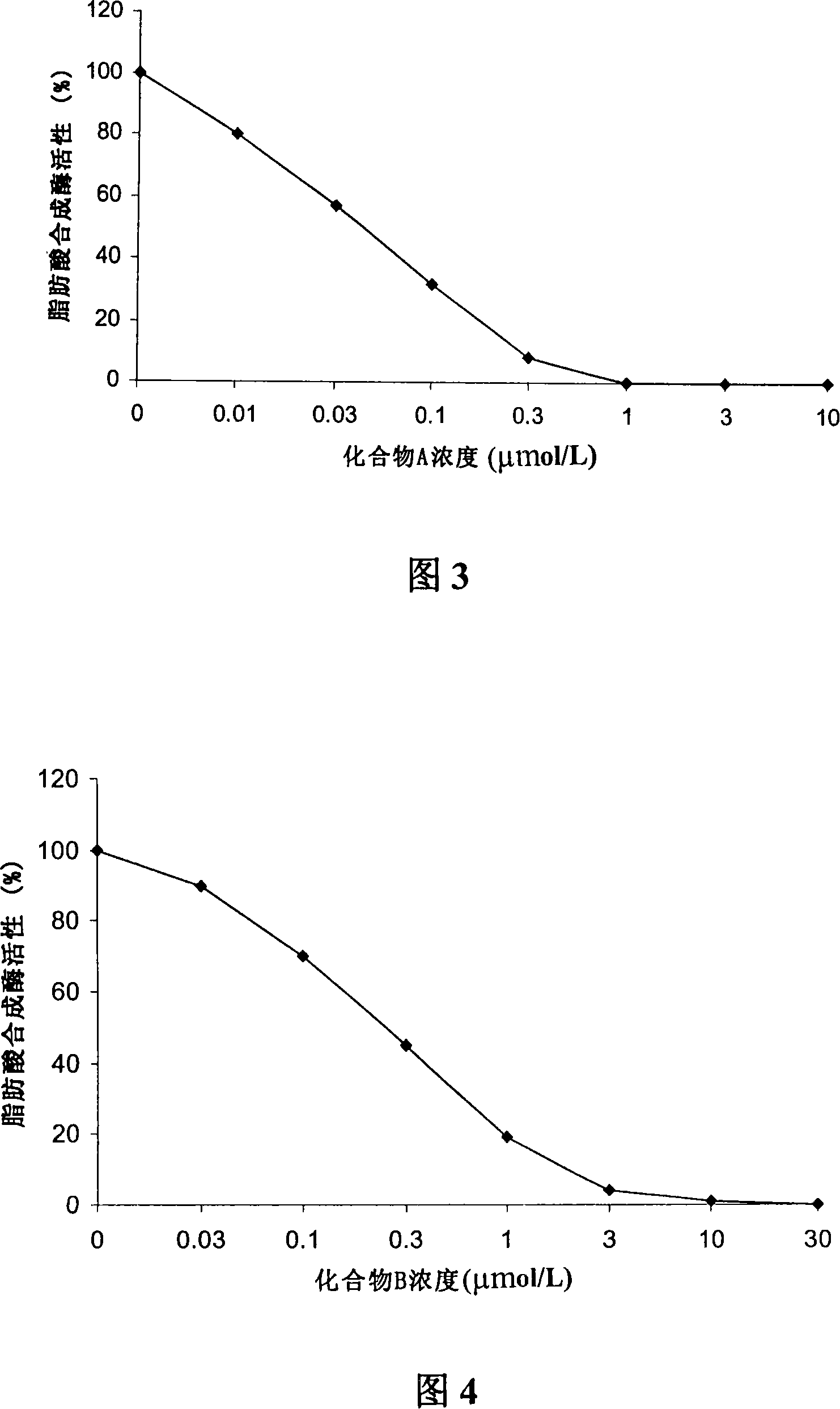
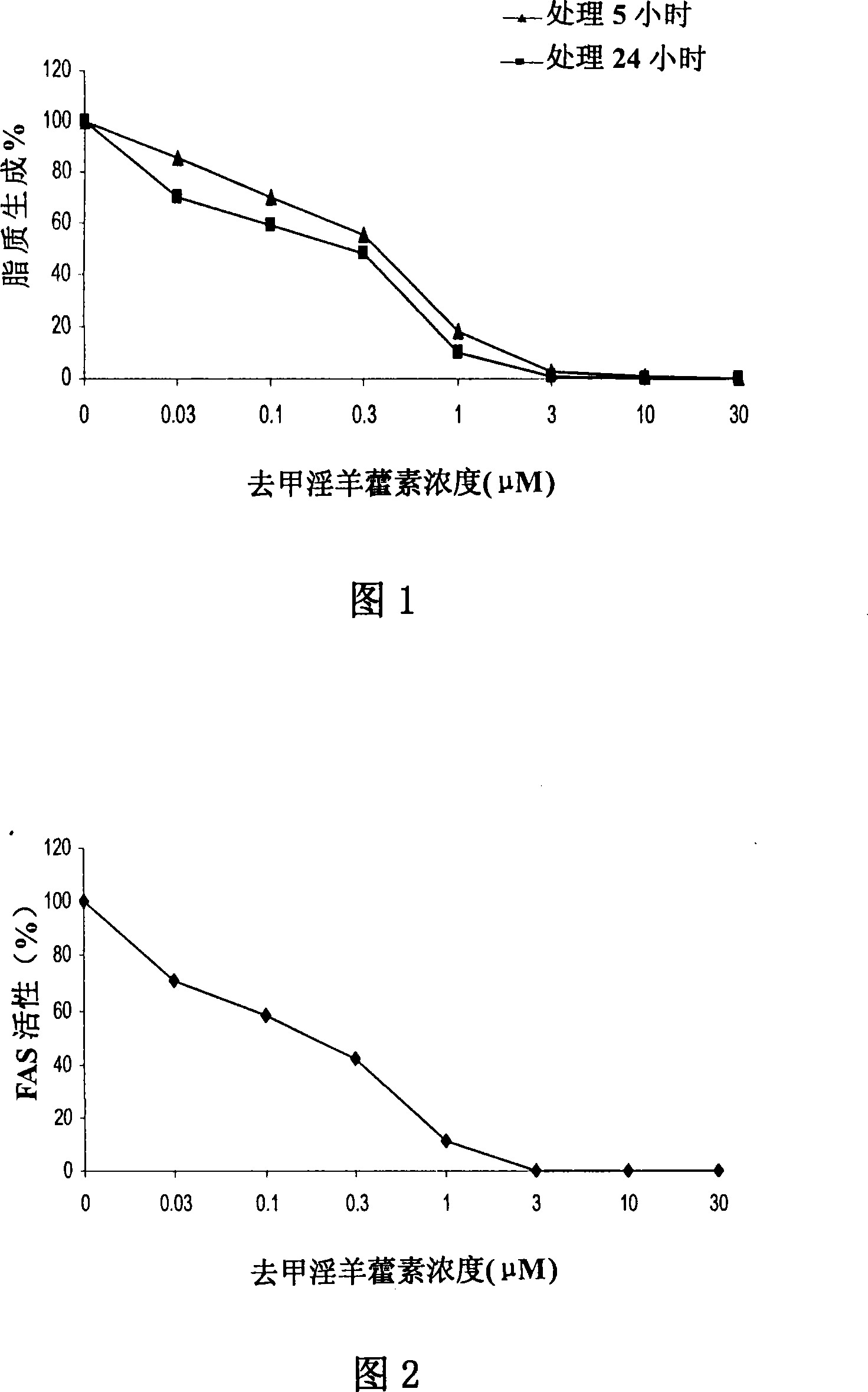
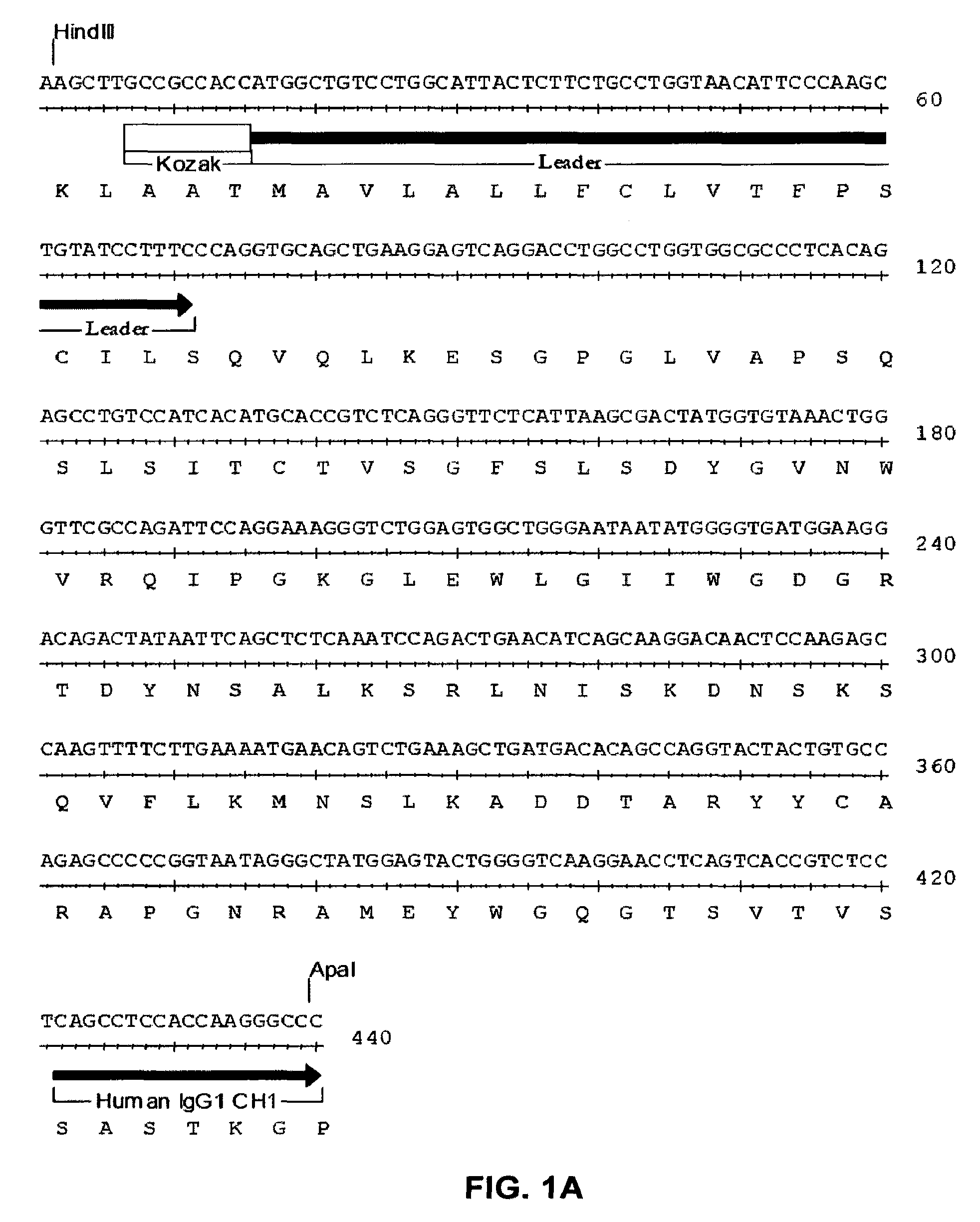
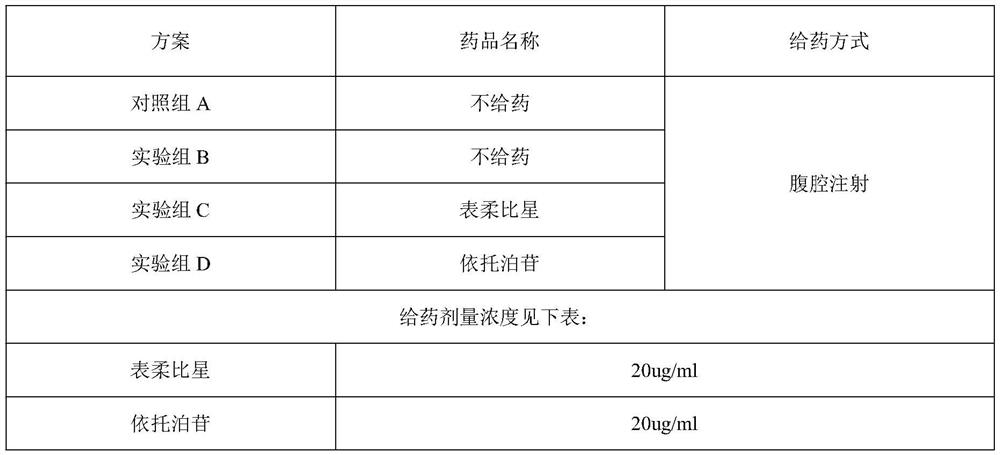
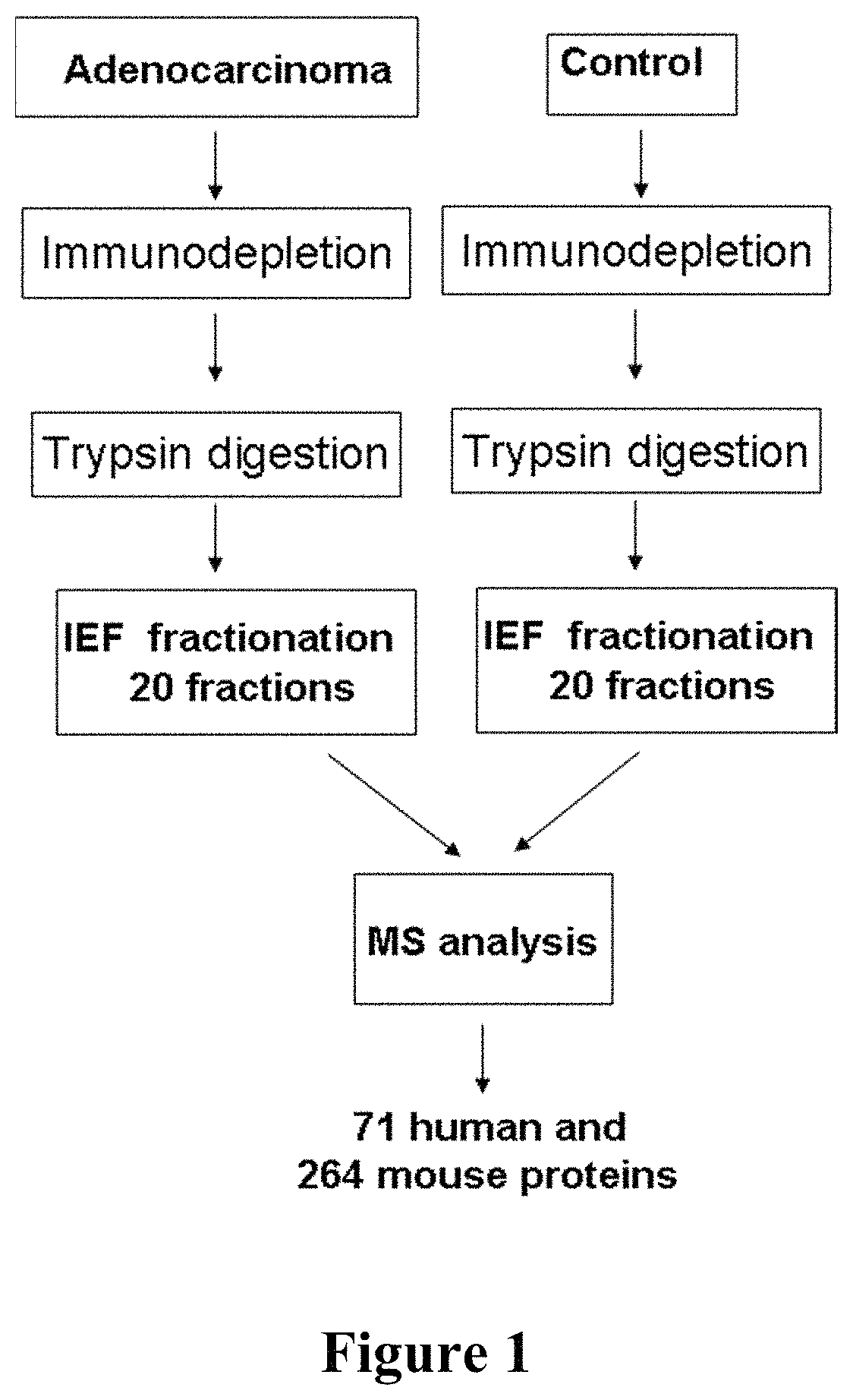
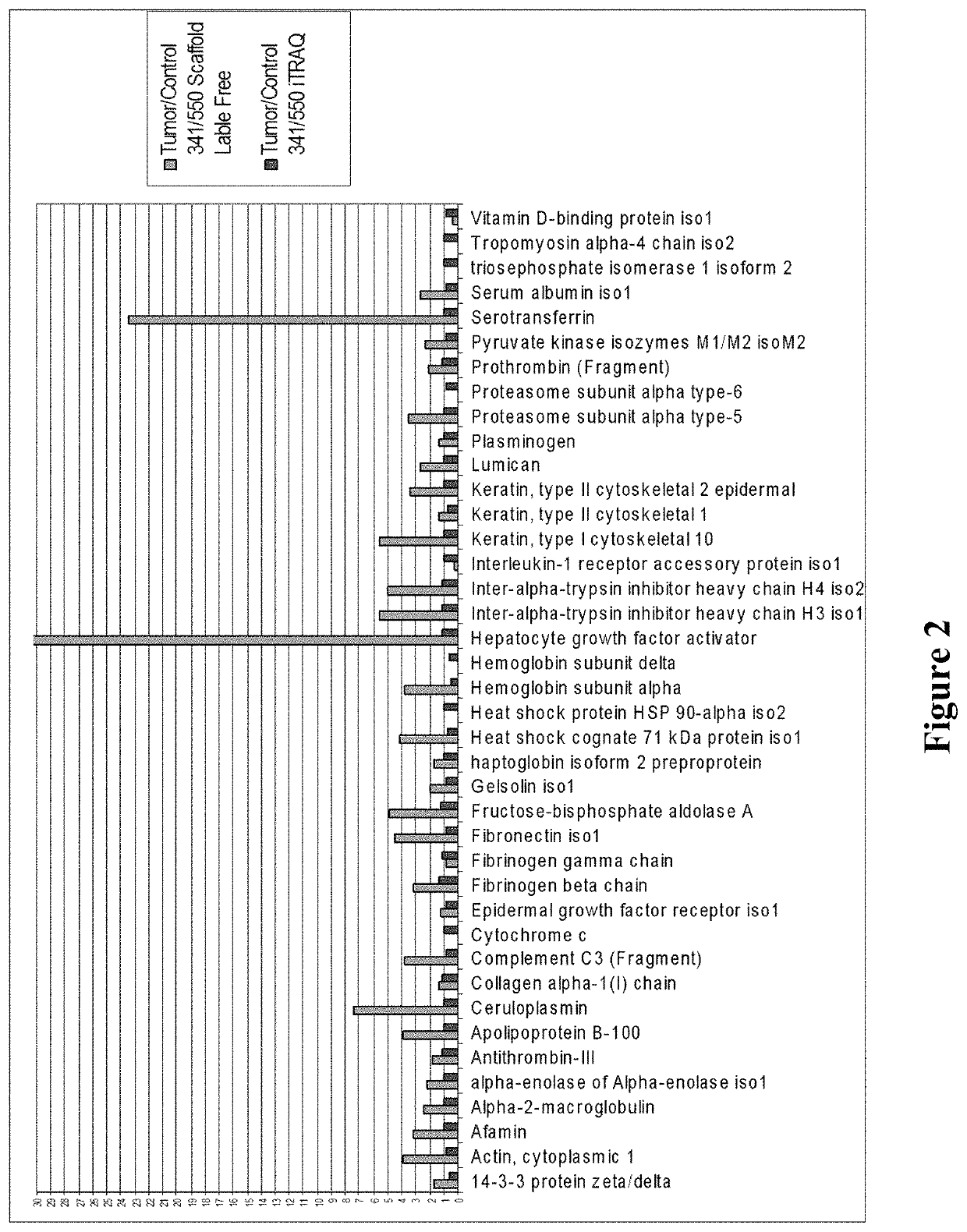
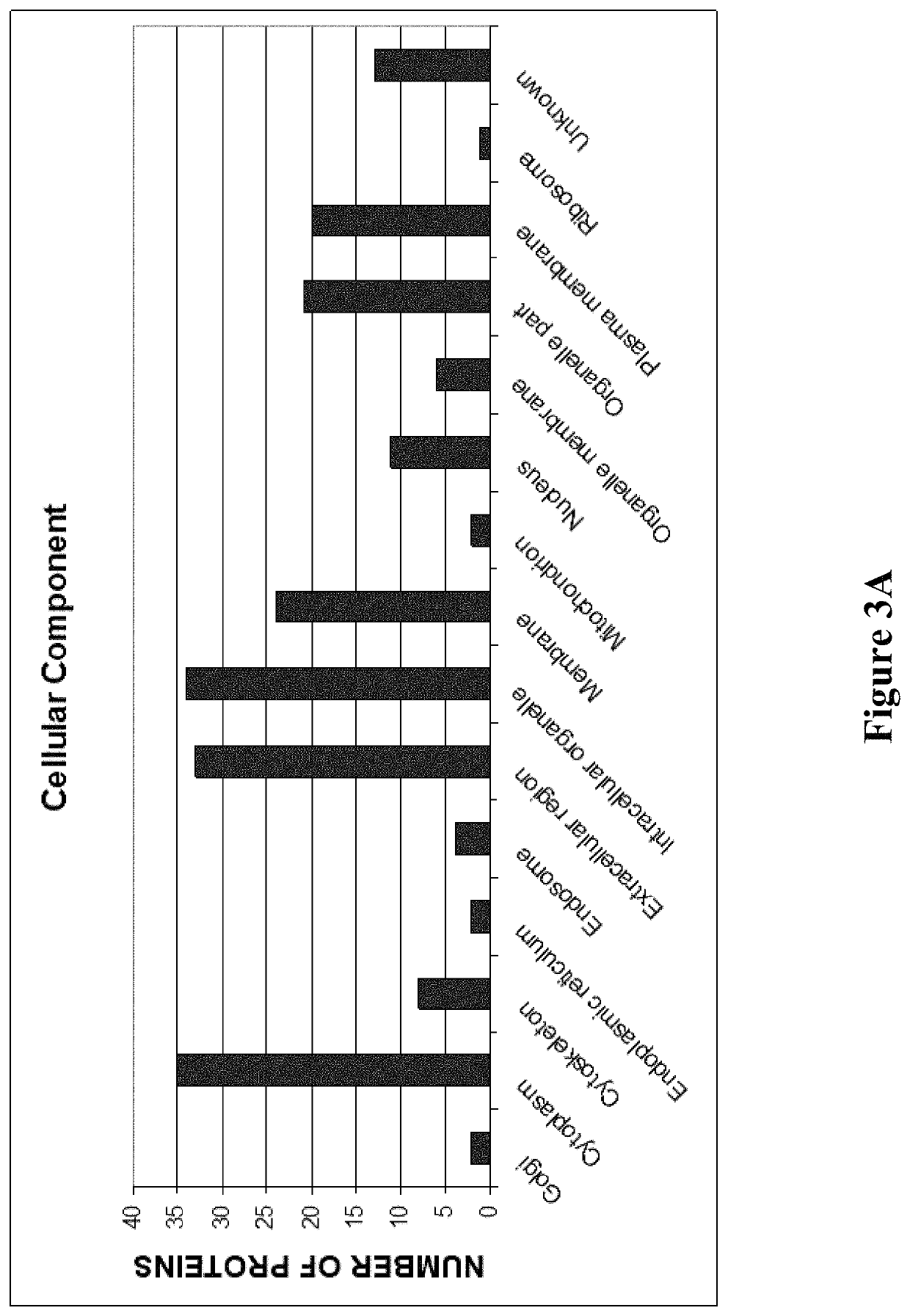
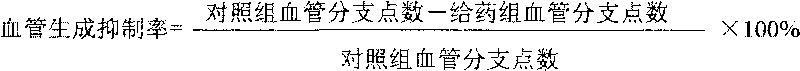Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Tumor transplantation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation of functional area of sea purse blood vessel growth inhibition factor 1 and use of the functional area of sea purse blood vessel growth inhibition factor 1 in medicaments for preventing and curing tumors
InactiveCN101724631AStrong specificityHigh expressionPeptide/protein ingredientsFermentationAbnormal tissue growthAcute toxicity testing
The invention relates to the preparation of a functional area of a sea purse blood vessel growth inhibition factor 1 and the use of the functional area of the sea purse blood vessel growth inhibition factor 1 in medicaments for preventing and curing tumors. In the invention, a gene engineering technique is used to realize the cloning, expression and recombination of the sea purse blood vessel growth inhibition factor 1; the recombinant functional fragment of the sea purse blood vessel growth inhibition factor 1 has the bioactivities for resisting the growth of blood vessels, tumor growth, tumor transplantation, acute toxicity tests and stability tests; and the functional area of the sea purse blood vessel growth inhibition factor 1 can be mixed with or dissolved in pharmaceutically acceptable carriers to prepare the medicaments for curing various tumors. The functional area of the sea purse blood vessel growth inhibition factor 1 has high action specificity, has the characteristics of easy expression, low degradation rate and the like of gene engineering medicaments and has the effects of inhibiting blood vessel growth, tumor growth and tumor transplantation; the provided gene engineering technique can realize the industrial production of the functional area of the sea purse blood vessel growth inhibition factor 1; and the functional area of the sea purse blood vessel growth inhibition factor 1 prepared by the gene engineering technique can be used in the preparation of medicaments for inhibiting blood vessel growth and preventing and curing tumors.
Owner:GUANGDONG OCEAN UNIVERSITY
3-methoxylflavonoid compound, preparation method and application thereof
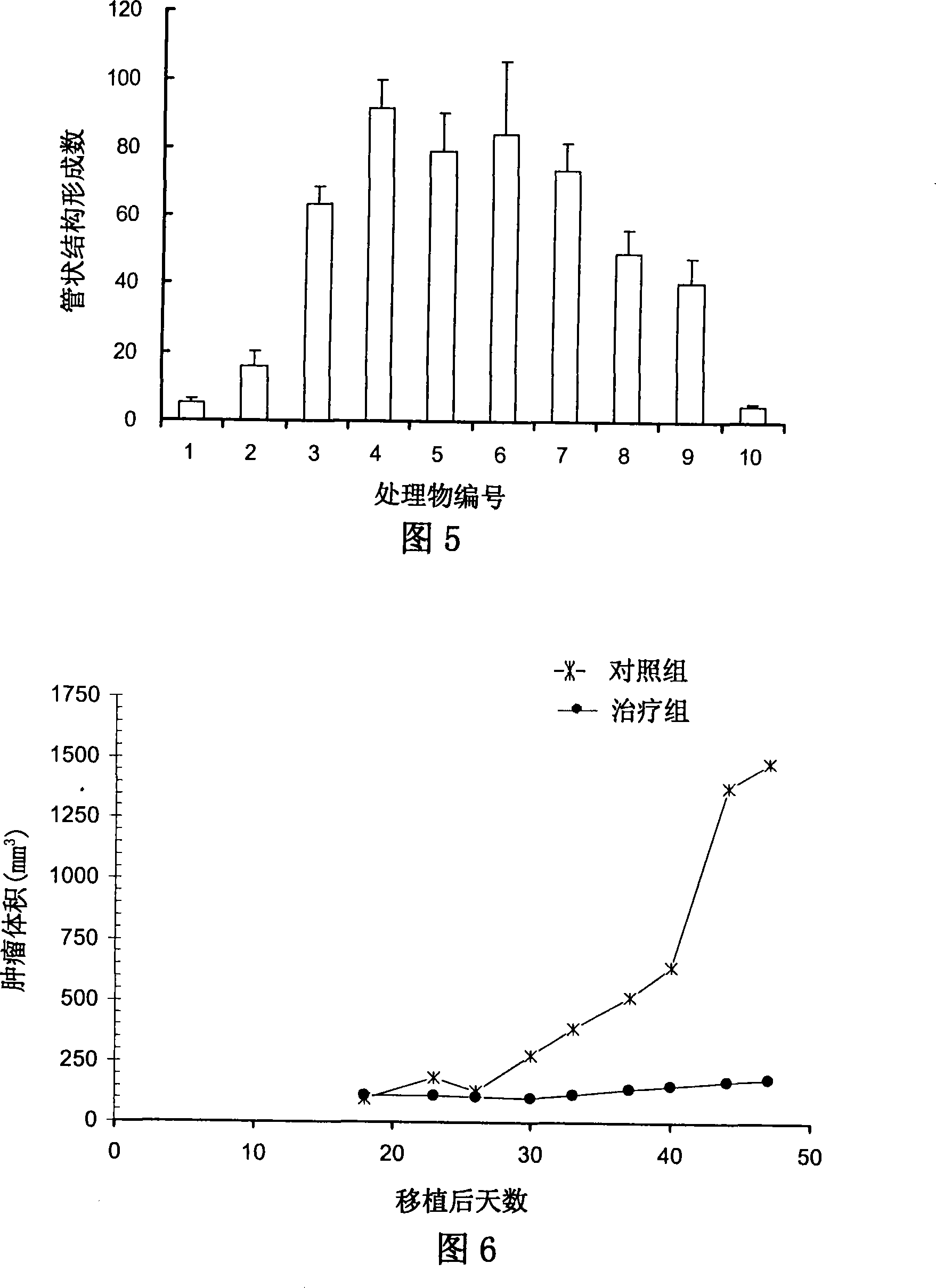
A 3-methoxy-flavone compound comprises 5,7-dihydroxy-8-(3,3-dimethyl diallyl)-3,3', 4'-trimethoxy flavone and 5, 7-2 dihydroxy-8- (3,3-dimethyl allyl)-3,4'-dimethoxy flavonoe. harmacological test results prove that: the 3-methoxy-flavone compound is an effective fatty acid synthase inhibitor, which shows the broad-spectrum anti-tumor effect in the cytotoxicity tests of various tumor cell lines and has strong tumor growth inhibiting effect on human prostate cancer cell LnCAP, human breast cancer cell ZR-75-1, human lung cancer cell NCI-H23 or human colon cancer cell HCT-116 when applied to human tumor transplant nude mice model tests. In addition, the 3-methoxy-flavone compound reveals no toxicity in mice acute toxicity tests, which then can be used as anti-tumor drug and is a new broad-spectrum anti-tumor drug with great development prospects.
Owner:殷正丰 +2
Anti-tumor medicine
InactiveCN101103973AOrganic active ingredientsAntineoplastic agentsAbnormal tissue growthProstate cancer cell
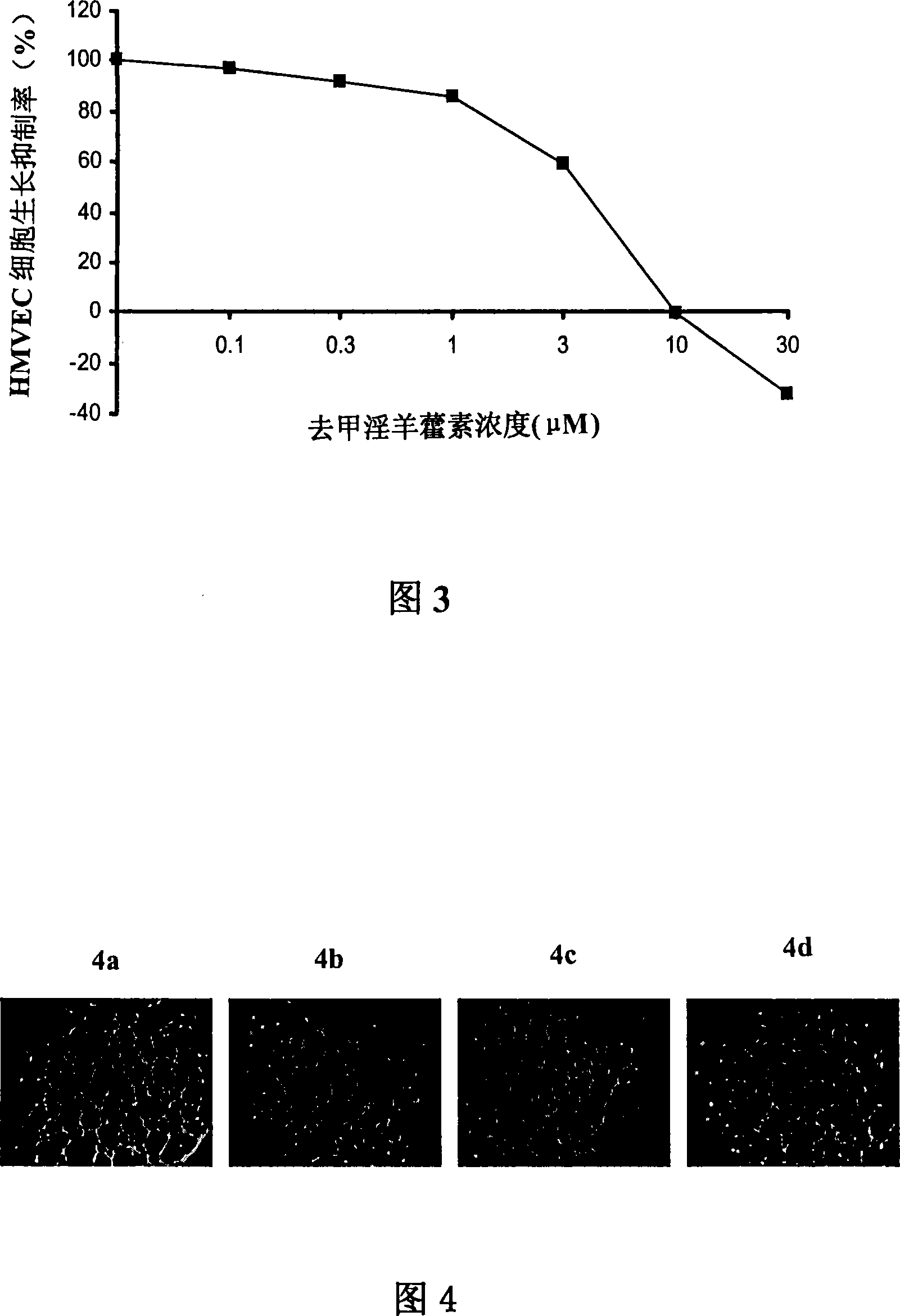
Disclosed is an anti-tumor drug with nor-icariin as the effective ingredient. Pharmacological test effect proves that the nor-icariin has the activity to inhibit the production of cellular lipid, can reduce the production of cellular fatty acid by inhibiting the activity of FAS, has a function of inhibiting the production and causing withering of microvascular endothelial cells, shows a broad-spectrum anti-tumor function in a cytotoxicity test of thirty-three tumor cell strains of a human body, and shows a function in strongly inhibiting the production of tumor, such as prostate cancer cells LnCAP, lung cancer cells NCI-H23 and colon cancer cells HCT-116 of a human body in a tumor transplantation nude mice model test. A pharmacokinetics test of the nor-icariin in the body of a little mouse proves that the nor-icariin can be kept with a certain concentration, good for better anti-humor effect. The acute toxicity test of the nor-icariin in the body of the mouse shows nontoxicity; therefore the nor-icariin is proved to be a newly-developed broad spectrum anti-humor drug with broad development prospect.
Owner:殷正丰 +1
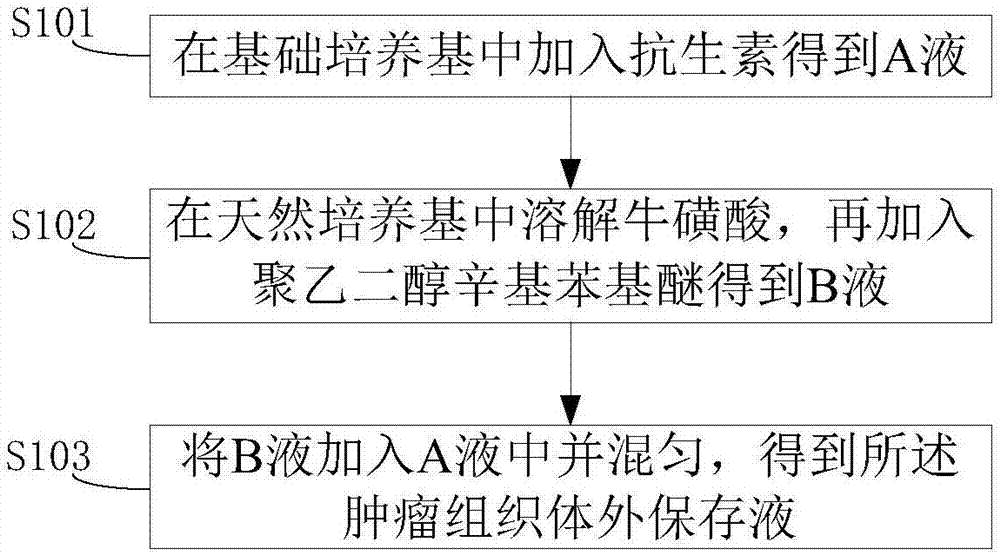
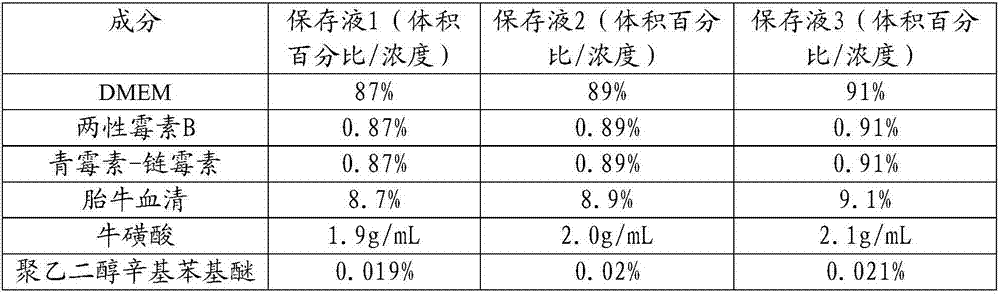
Tumor tissue in-vitro preservation liquid and preparation method thereof
InactiveCN107094752AMaintain biological activityProtectDead animal preservationPolyethylene glycolAntibiotic Y
The invention discloses tumor tissue in-vitro preservation liquid and a preparation method thereof. The method comprises the following steps: adding antibiotics into a basic culture medium to obtain a solution A; dissolving taurine into a natural culture medium and adding polyethylene glycol octylphenol ether to obtain a solution B; adding the solution B into the solution A and uniformly mixing to obtain the tumor tissue in-vitro preservation liquid. The in-vitro preservation liquid prepared by the method can be used for keeping the bioactivity of tumor tissues in an in-vitro preservation process, prolonging the in-vitro preservation time of the tumor tissues and improving the success rate of tumor transplantation.
Owner:南通普惠精准医疗科技有限公司
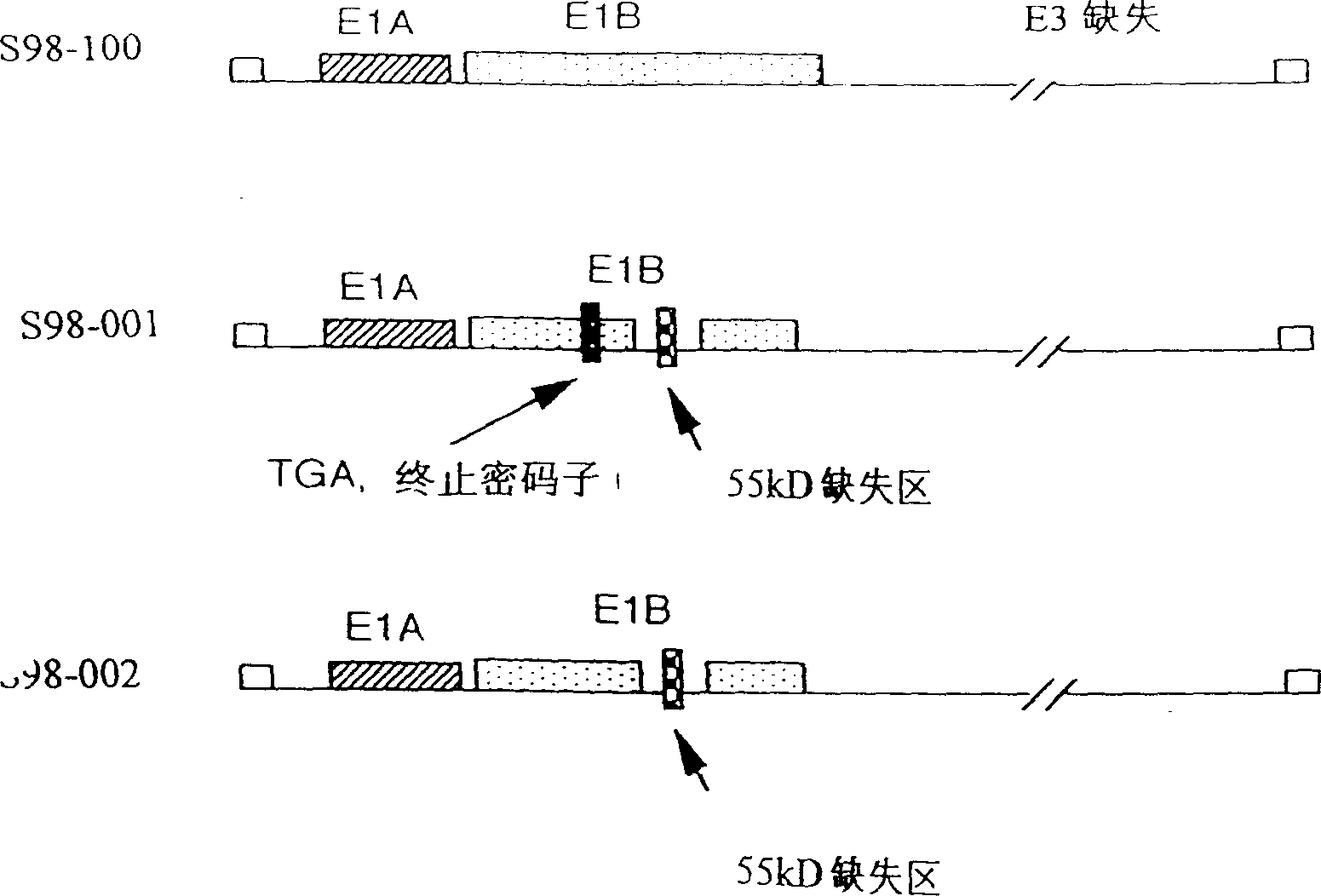
Gene engineering adenovirus and it application
InactiveCN1110553CViruses/bacteriophagesAntibody medical ingredientsAbnormal tissue growthHuman tumor
The present invention construts two kinds of gene engineering adenovirus capable of being replicated selectively in human tumor cells with P53 function deficiency for ultimately killing tumor cells. Its tumor resisting curative effect is determined through the human tumor model transplanted to mouse body and test data shows that the gene engineering adenovirus can be used in treating specific tumor.
Owner:SHANGHAI SUNWAY BIOTECH +1
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:AERES BIOMEDICAL
Digestive tract tumor PDX model and construction method of standardized model library
PendingCN113142135AFacilitate adjuvant therapyProcess specificationCompounds screening/testingAnimal husbandryDigestive canalTumor therapy
The invention provides a digestive tract tumor PDX model and a construction method of a standardized model library. The method comprises the following steps: conducting sampling, treating a collected peripheral blood sample of a digestive tract tumor, obtaining tumor cells, conducting treating and culturing, stably passing the tumor cells to n generations, constructing a digestive tract tumor PDX model and evaluating the model. A Chinese population human-derived digestive tract tumor transplantation animal model and a corresponding primary tumor cell line are established in a digestive tract tumor PDX standardized model library, an important in-vivo model is provided for biological research of tumors, searching of diagnostic markers and drug screening, randomness and blindness of drug use are avoided, disturbance by factors such as original tissue samples is avoided, the digestive tract tumor PDX model can be stably subcultured to three generations or more, and the construction of the digestive tract tumor PDX model standard sample library provides bioinformatics for exploring new targets and searching and analyzing clinical characteristics of digestive tract tumors, and assists in research of a fatal mechanism of digestive tract tumors and treatment of patient tumors.
Owner:SHANDONG PROVINCIAL HOSPITAL AFFILIATED TO SHANDONG FIRST MEDICAL UNIVERSITY
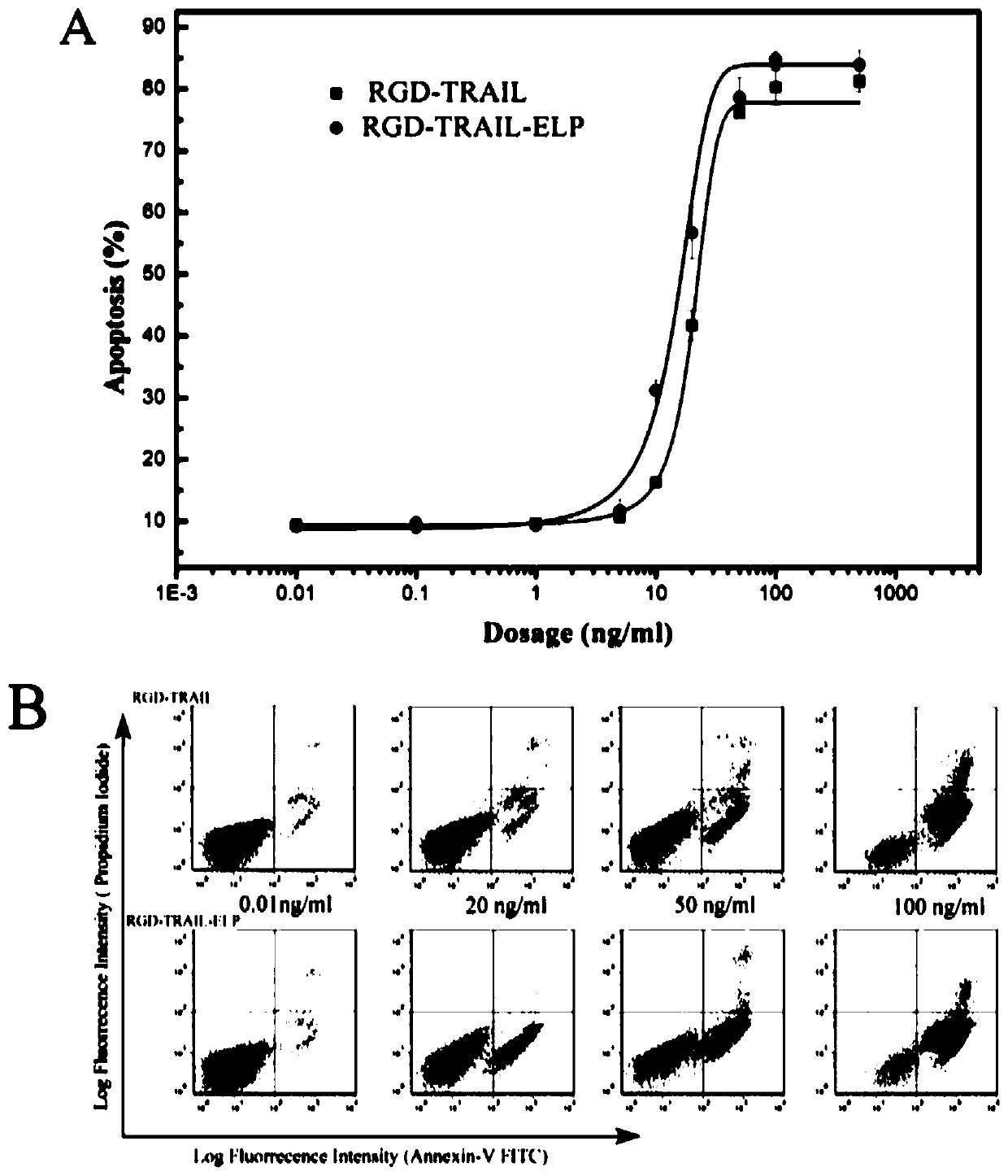
Fusion protein comprising tumor necrosis factor-related apoptosis-inducing ligand, preparation method of fusion protein, and nanoparticles formed by self-assembled fusion proteins
ActiveCN106519040AIncreased apoptotic activityAchieving soluble expressionBacteriaPeptide/protein ingredientsIntraperitoneal routeEscherichia coli
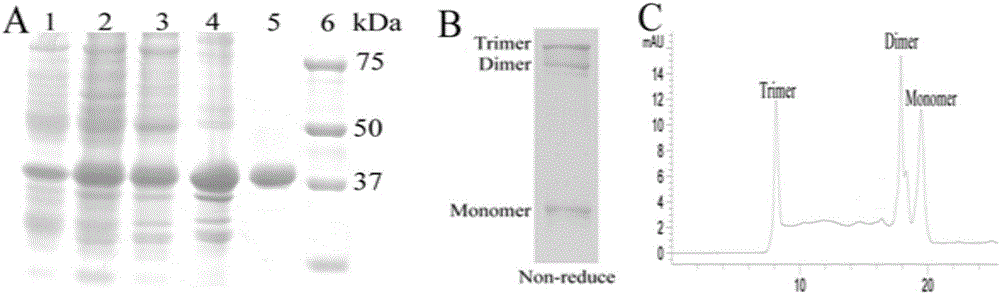
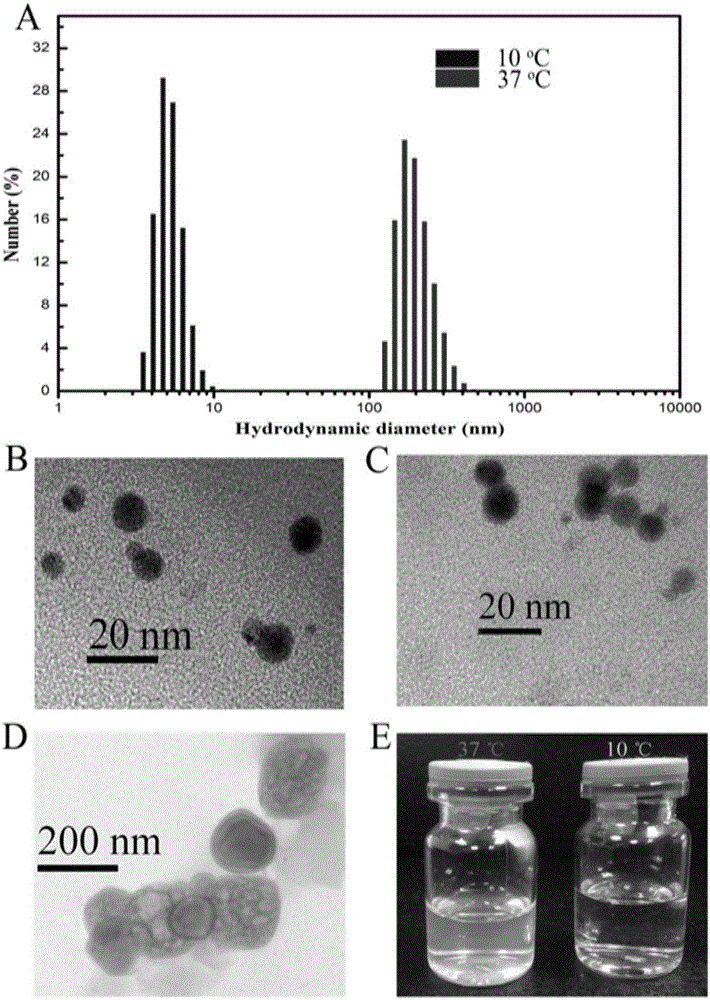
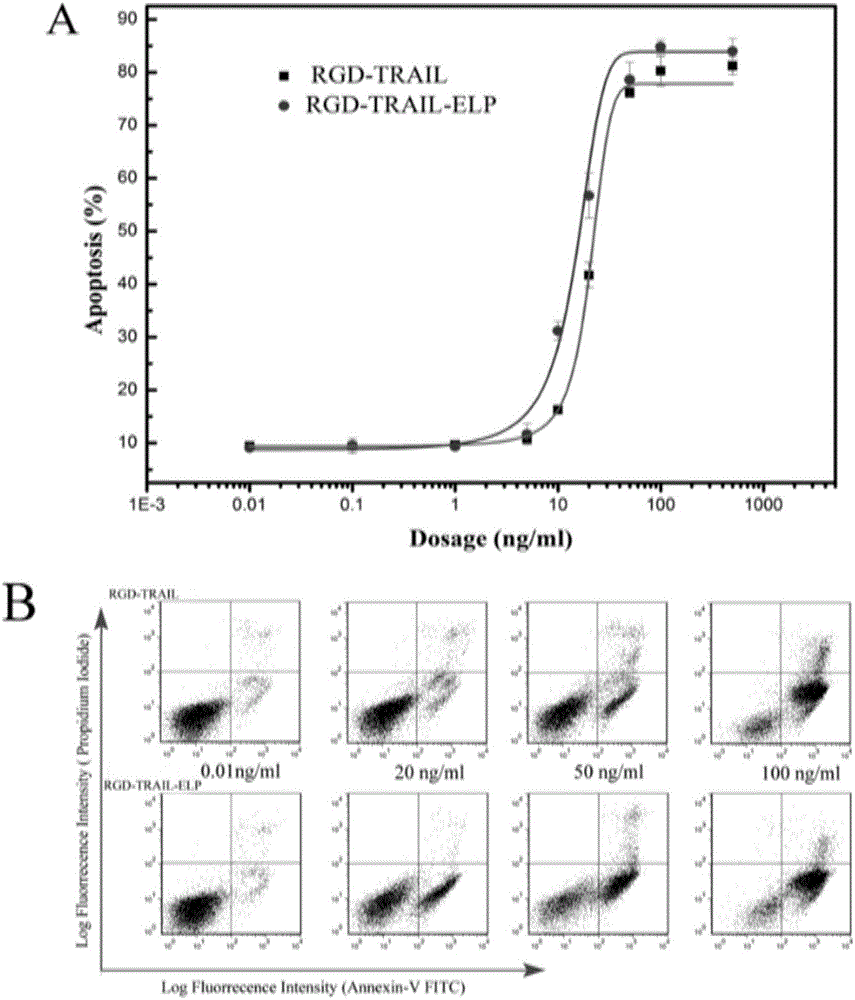
The invention belongs to the field of bio-pharmaceuticals and particularly relates to expression, purification and self-assembly of TRAIL variant proteins. A recombinant protein is expressed by plasmids comprising the TRAIL fusion proteins in escherichia coli, and the recombinant protein with high activity is produced. The recombinant proteins adopt a centrifugal mode for simple and rapid purification. The purified proteins are self-assembled into particles being 200 nanometers under the physiological conditions. After intravenous injection to a tumor transplantation mouse, the recombinant protein is effectively gathered in the tumor location of the mouse. The recombinant protein is injected into the tumor transplantation mouse in an intraperitoneal injection mode, and tumor growth is effectively suppressed. The recombinant protein subjected to simple process purification is used for oncotherapy.
Owner:NANJING UNIV
Method for establishing fluorescent orthotopic transplantation model for subcutaneous transferred organ cancer
The invention relates to a method for establishing a model of fluorescence orthotopic transplantation of subcutaneous metastasis organ cancers, which is characterized in that human colon adenocarcinoma cell strains or cancer cell strain green fluorescent proteins (GFP) of corresponding organs are selected; GFP plasmids carry out transfection on cancer cell strains of the organs via retrovirus pLPCX so that the cancer cell strains of the organs contain GFP expression plasmids; a GFP gene is connected with a pLPCX vector via a T4 ligase; a GFP-pLPCX vector is transfected to a PT67 package cell to obtain a Lovo cell; nude mice are selected and subjected to subcutaneous inoculation with Lovo cell suspension; tumors can be formed in about 4 weeks; orthotopic transplantation is carried out after green fluorescence is observed; a cut is opened on the skin and peritoneum; about 1cm cecum of the nude mice is taken out of the peritoneum; the gap between the cecum and the peritoneum is sutured and the cecum is transferred subcutaneously; the cut cecum is subjected to serosa; two fluorescence-carrying tumors with diameter of about 1mm are transplanted to the cecum via the suture and the skin is closed.
Owner:NANJING ORIGIN BIOSCI
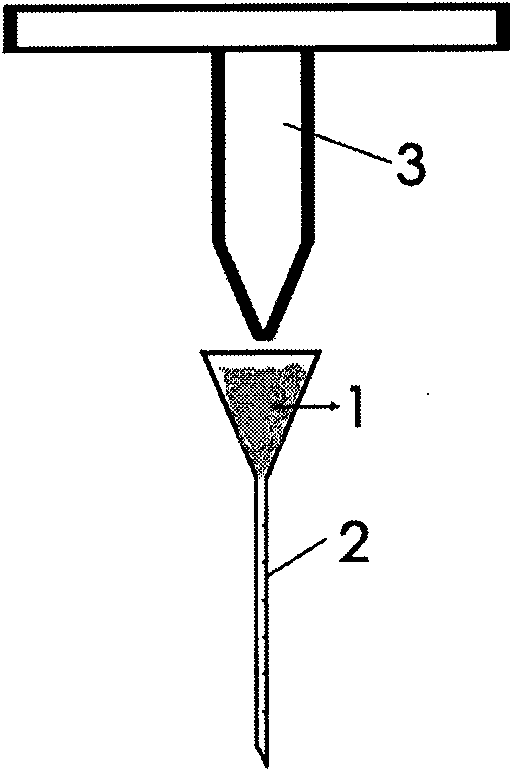
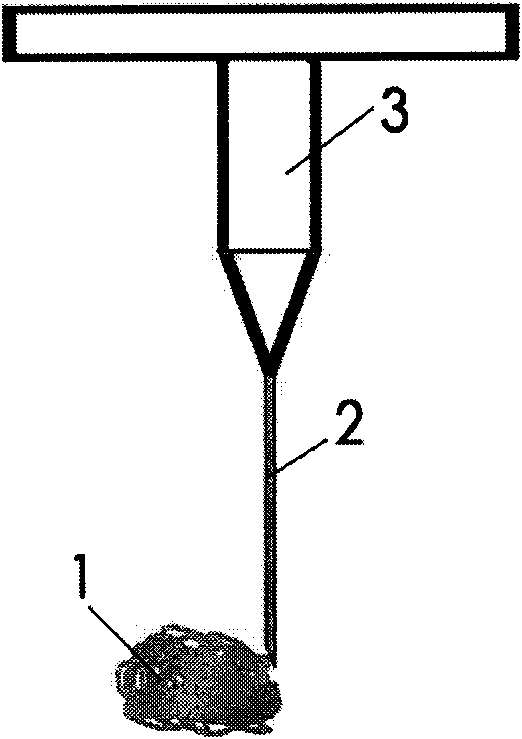
In-situ inoculating operation method for cerebral tumor tissue block animal and inoculating needle thereof
The invention discloses an in-situ inoculating operation method for a cerebral tumor tissue block animal. The method comprises the following steps: (a) taking a cerebral tumor tissue, and shearing the cerebral tumor tissue into tumor tissue particles; (b) placing the tumor tissue particles into a needle tube of a tissue block injection needle, and carrying out quantifiable indwelling control on the tumor tissue particles; (c) anaesthetizing the animal, dissecting the skin on the surface of the animal skull, and boring a hole on the animal cranial bone; (d) inserting the tissue block injection needle along a cranial bone hole, and slowly injecting the tumor tissue particles; and (e) slowly pulling out the needle, sealing the cranial bone hole by bone wax, and suturing the scalp. The method can ensure the original tissue structural property of an inoculated tumor tissue, provides a tumor model with high simulation degree for tumor research, can ensure the vitality of inoculated cells, has high tumor transplantation success rate, can realize quantification of cell number in the inoculated tumor tissue and positioning of inoculation in the tumor tissue animal brain, has short operation time, and can meet the requirement of needing large amount of animal models in once experiment.
Owner:THE SECOND HOSPITAL AFFILIATED TO SUZHOU UNIV
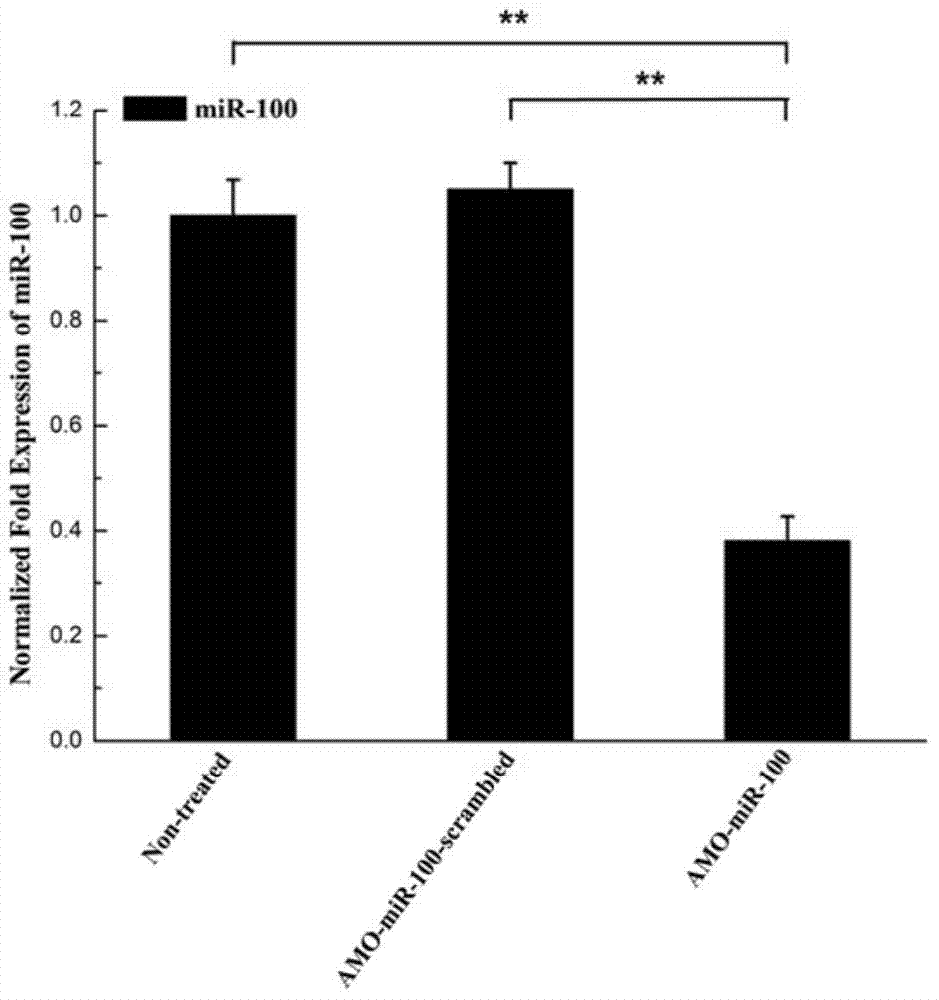
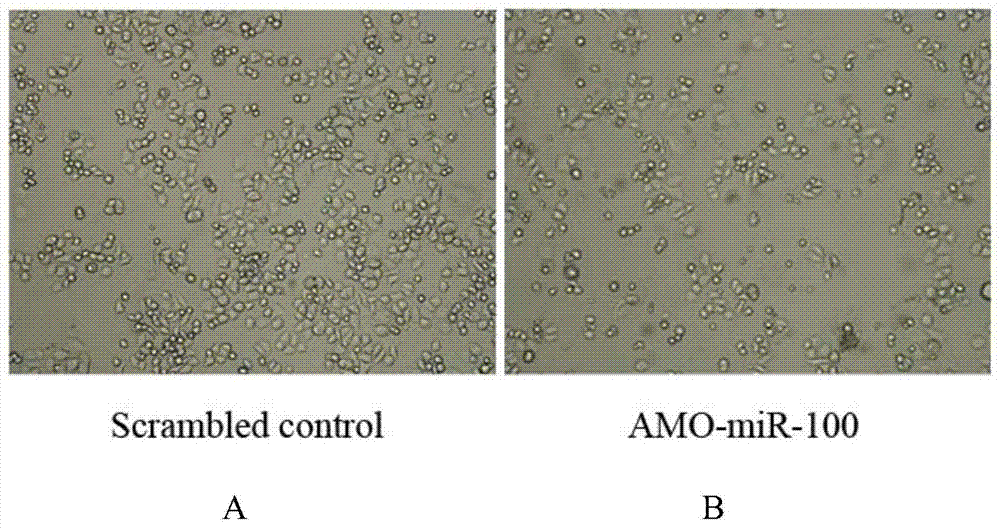
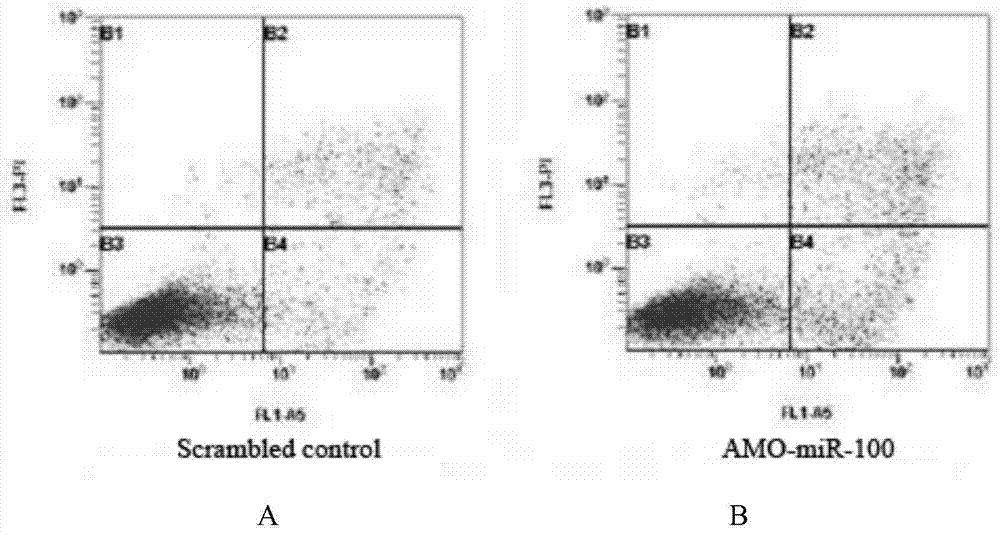
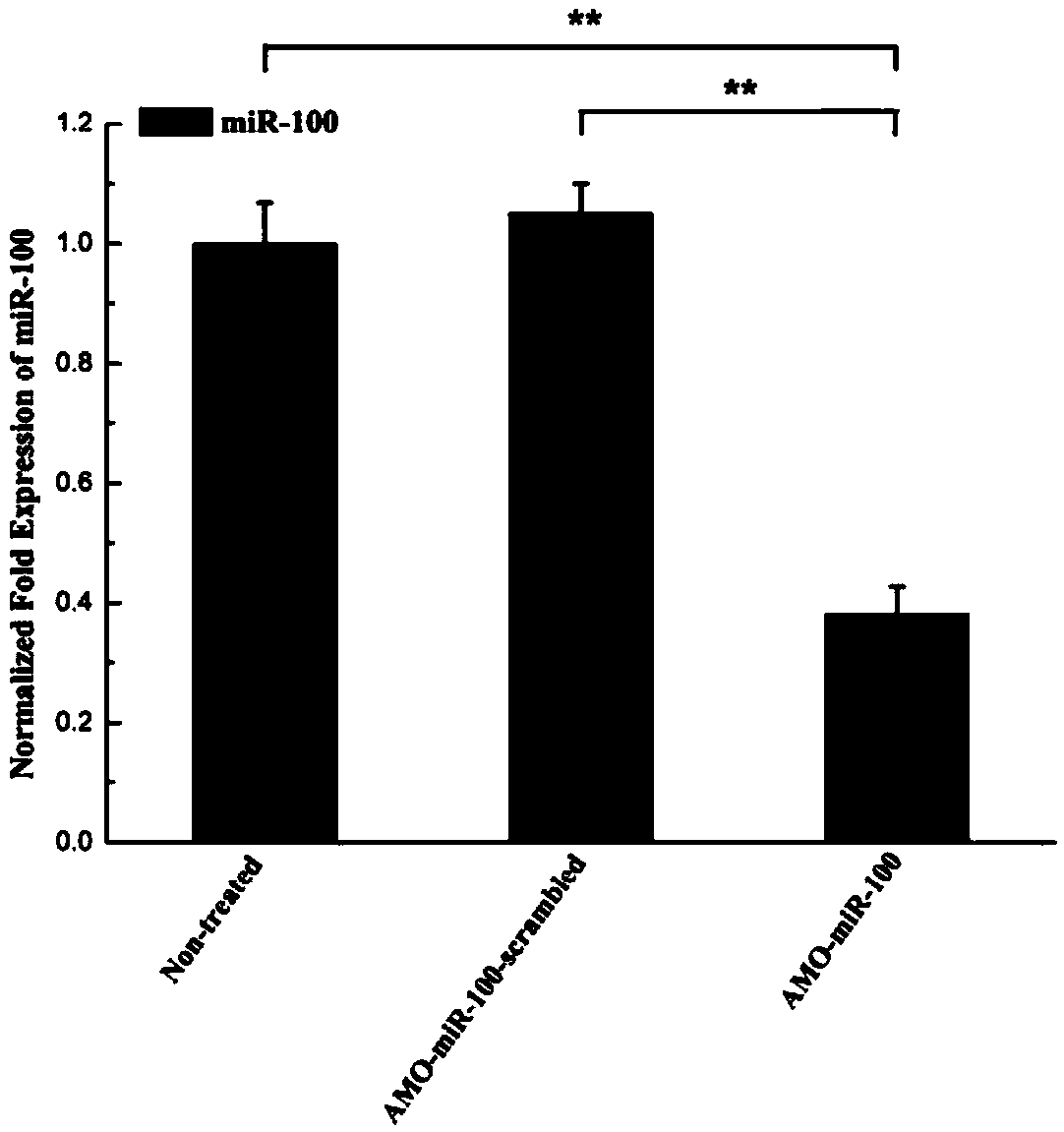
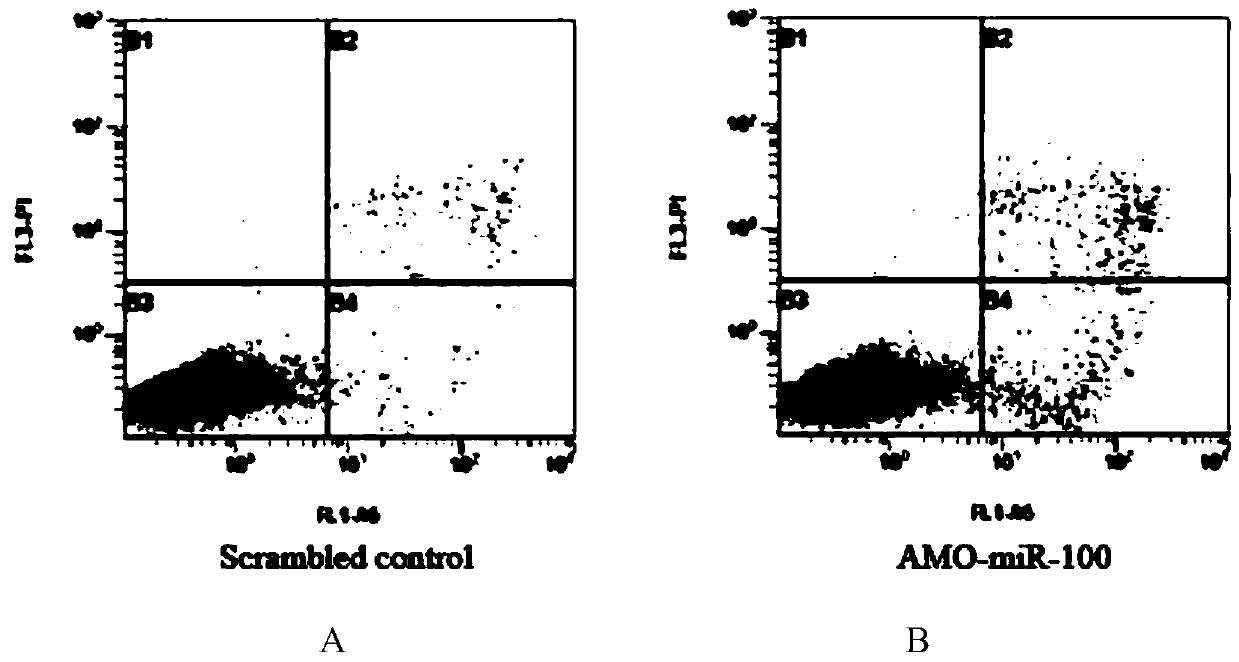
Application of miR-100 inhibitor in preparation of medicine for treating breast cancer
ActiveCN103893780AGrowth rate inhibitionInduce apoptosisGenetic material ingredientsAntineoplastic agentsAntisense nucleic acidTumor transplantation
The invention discloses application of a miR-100 inhibitor in preparation of a medicine for treating a breast cancer. The miR-100 inhibitor is a miR-100 antisense nucleic acid chain, and the sequence is 5'-TTCGGATCTACGGGTT-3'. The miR-100 inhibitor disclosed by the invention can induce apoptosis of tumor cells, growth of tumors in tumor transplanted mice can be effectively induced, the sensitivity of breast cancer cells on chemotherapy medicines is increased, the effect of chemotherapy is improved, and the miR-100 is a latent new target for the medicine for treating the breast cancer, and is a new path for medical therapy.
Owner:ZHEJIANG UNIV +1
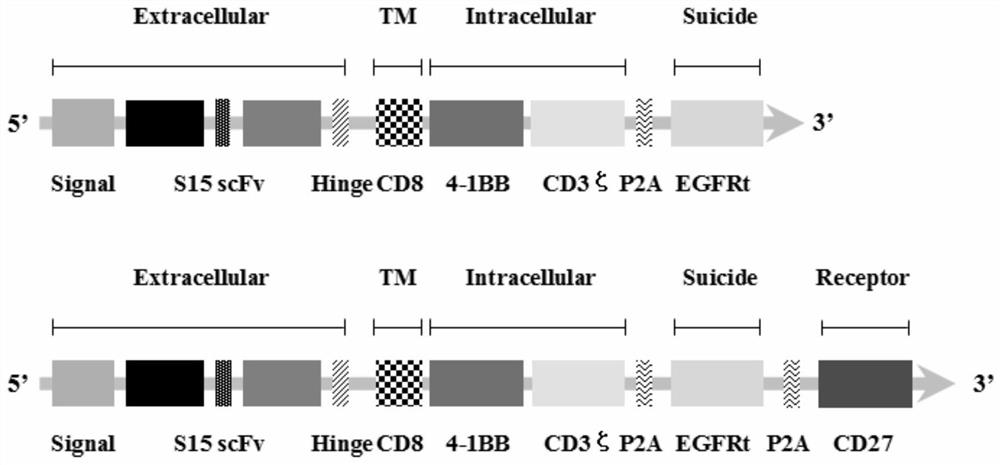
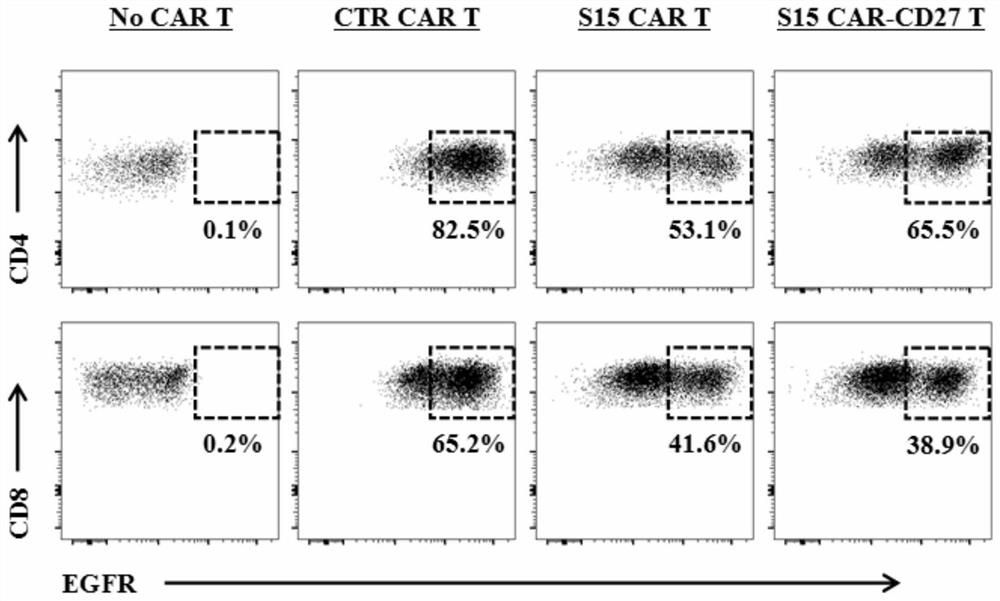
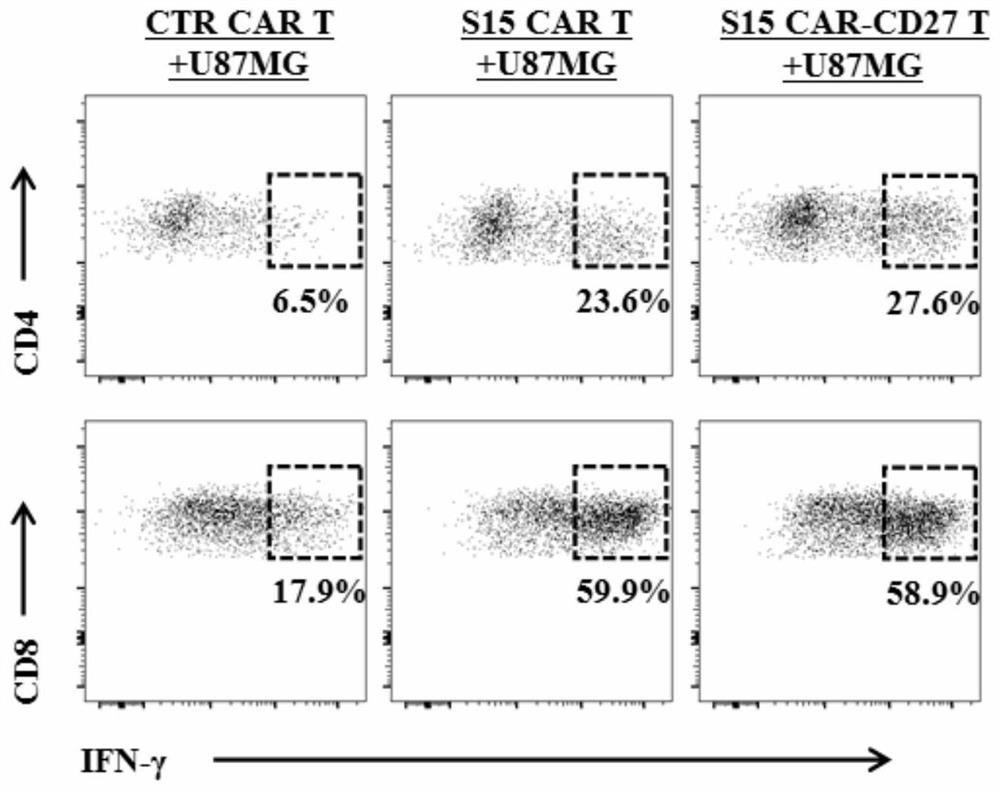
Chimeric antigen receptor T lymphocyte and application thereof to preparation of product for treating solid tumors
ActiveCN111875708ALift specific immune responseEnhance anti-tumor immune responseVirusesAntineoplastic agentsSingle-Chain AntibodiesAntiendomysial antibodies
The invention discloses a chimeric antigen receptor T lymphocyte and an application thereof to preparation of a product for treating solid tumors. A chimeric antigen receptor in the chimeric antigen receptor T lymphocyte sequentially comprises a human CD8 lead peptide, an anti-Siglec-15 single-chain antibody, a human CD8 hinge transmembrane region, a human 4-1BB intracellular region, a human CD3 zeta intracellular region, a self-cleavage peptide, a CSF2Ra signal peptide, an EGFRt protein, a self-cleavage peptide and a human CD27. Experiments prove that the chimeric antigen receptor T lymphocyte provided by the invention highly expresses IFN gamma and CD107a, has a strong killing function on Siglec-15 positive tumor cells, and has killing efficiency of more than 80% under the condition thatthe effect-target ratio is 1: 1. A tumor transplantation model experiment shows that the chimeric antigen receptor T lymphocyte also has a strong killing function on Siglec-15 positive tumor cells inan animal body.
Owner:CARBIOGENE THERAPEUTICS CO LTD
Delivery system based on miRNA-34a micromolecule regulator and preparation method and application of delivery system
InactiveCN105534899AGood biocompatibilityPromote degradationOrganic active ingredientsPharmaceutical non-active ingredientsEngineeringChalcone
The invention relates to a nanometer delivery system based on miRNA-34a micromolecule regulator and a preparation method and application of the delivery system. The delivery system comprises a nanometer delivery system body and the miRNA-34a micromolecule regulator loaded on the nanometer delivery system body. Polyethyleneimine-polycaprolactone nanomicelles modified by carboxymethyl glucosan are used as carriers, and the miRNA-34a micromolecule regulator is regulated from a hydrophobic small molecule compound 2'-hydroxyl-2,3,4',5,6'-pentamethoxyl chalcone which has liver-cancer-resistant activity and is also named as Rubone. By means of the delivery system, Rubone can be entrapped in a high-efficiency mode, the physicochemical property of Rubone is improved, the miRNA-34a micromolecule regulator can be delivered into liver cancer cells without using organic solvent, expression of miRNA-34a with the tumor restraining function in the cells is improved, proliferation of liver cancer cells can be effectively restrained, and it is further verified through a naked mouse liver cancer tumor transplantation model that growth of liver cancer can be remarkably retrained by injecting the nanomicelles in caudal veins.
Owner:BEIJING UNIV OF TECH
Preparation method of Rag1 gene defect animal model and application thereof
ActiveCN110564773ANo leaksGuaranteed success rateHydrolasesStable introduction of DNAImmunodeficiencyWilms' tumor
The invention provides a preparation method of a Rag1 gene defect animal model, which utilizes a gene editing technology to destroy a Rag1 gene coding region of a mouse, detects the model through immune system index evaluation and pathological histological examination, and verifies that the obtained mouse model is successfully constructed. The immunodeficiency model is helpful for research in thefields of tumor transplantation, immunology, inflammation and the like.
Owner:GEMPHARMATECH CO LTD
Method of cancer diagnosis, progression and response to therapy using a primary xenograft mouse model for cancer serum biomarker discovery
Owner:H LEE MOFFITT CANCER CENT & RES INST INC
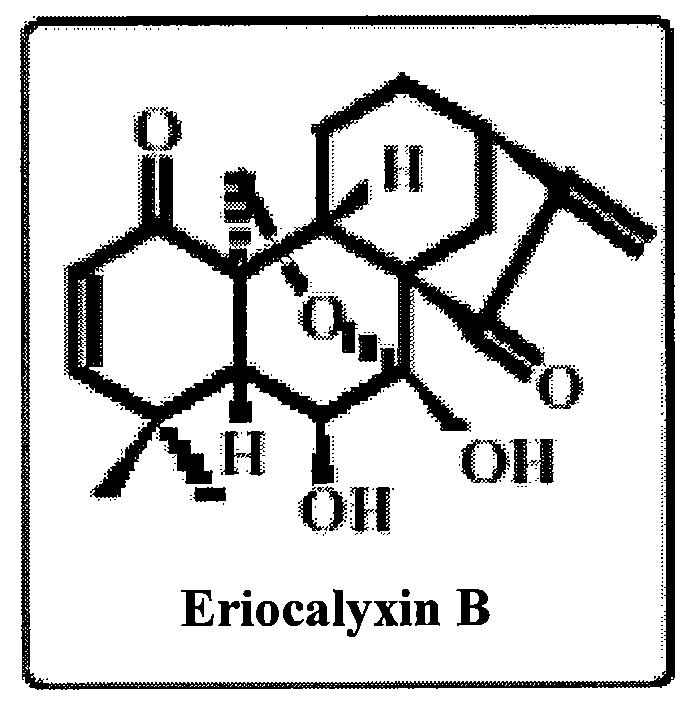
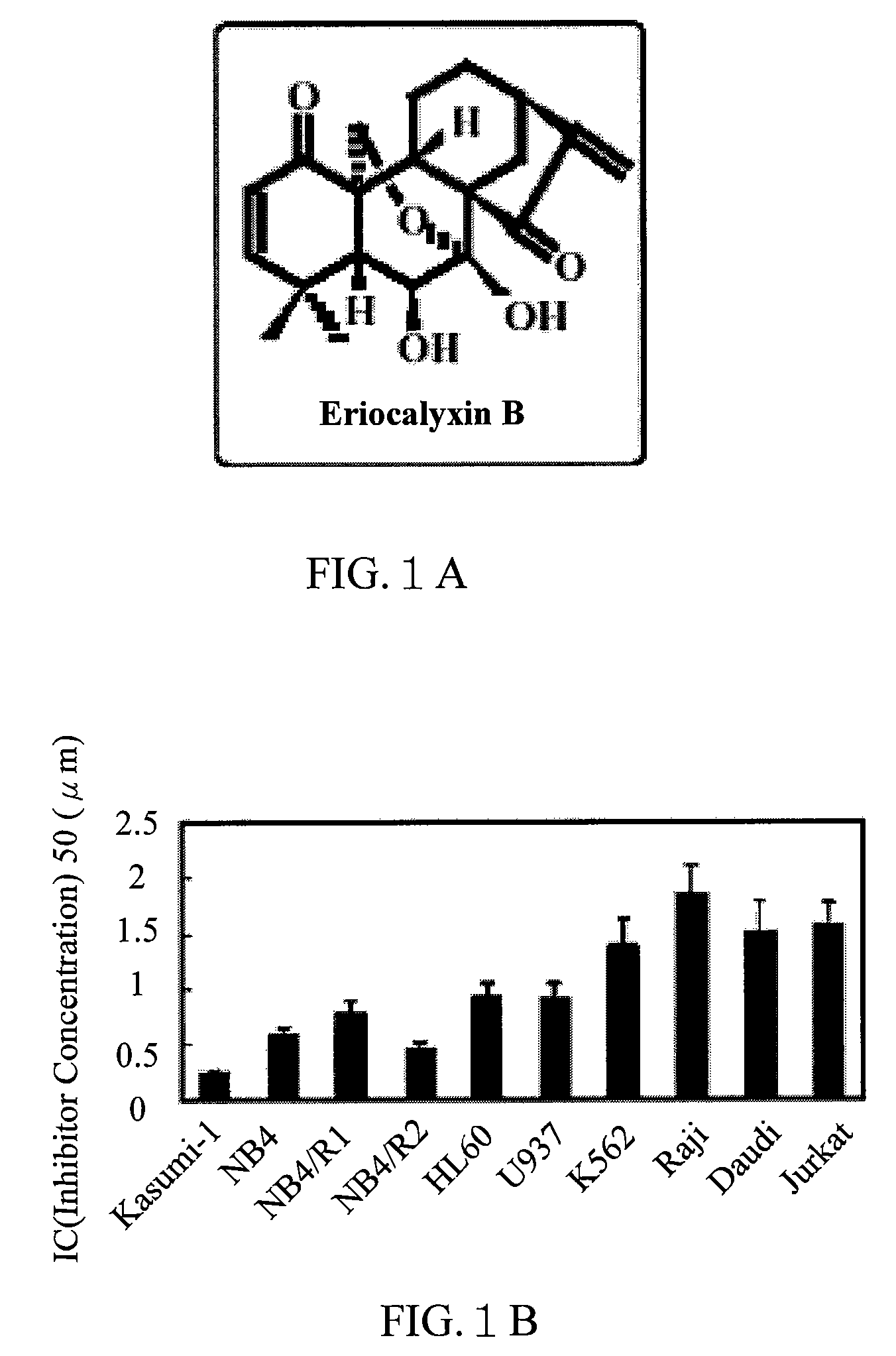
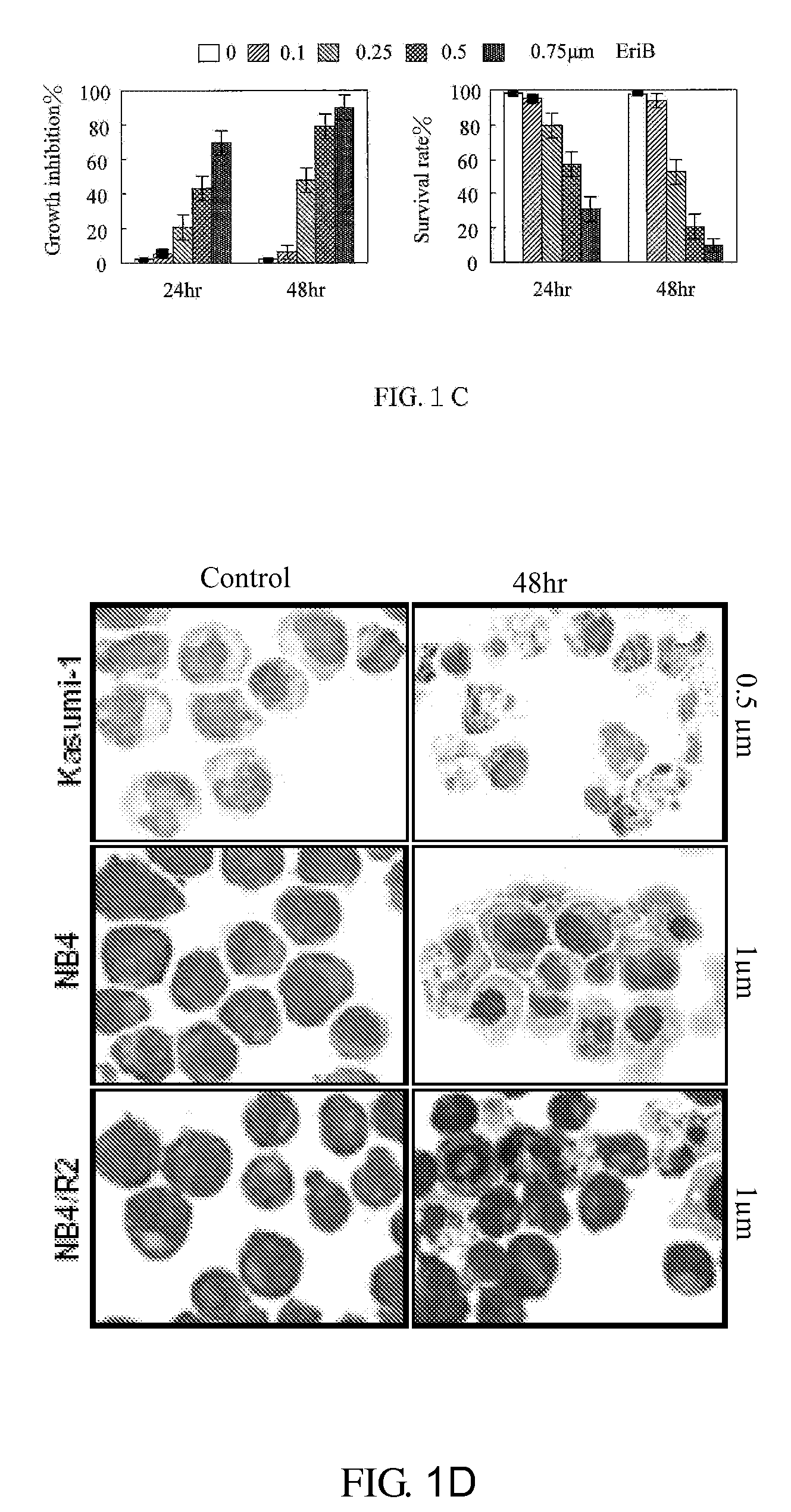
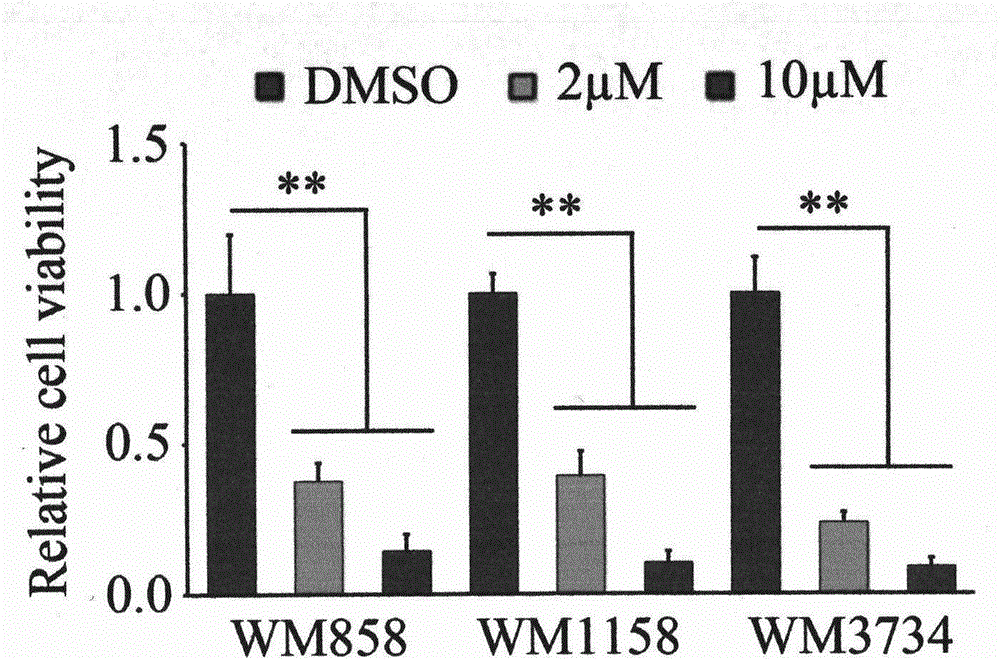
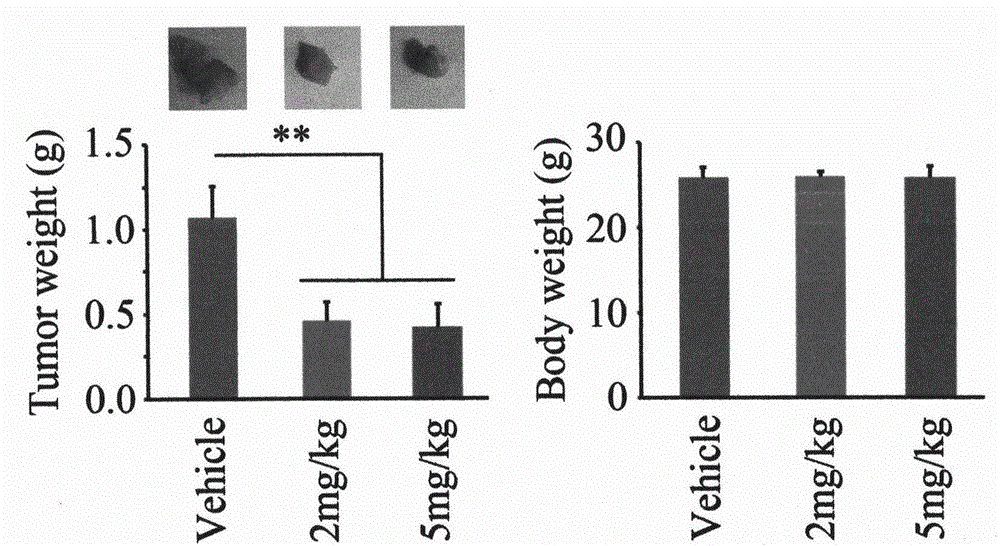
Application of Eriocalyxin B in the Manufacture of Medicaments For Treating Leukemia
InactiveUS20080300299A1Promote cell survivalLower the thresholdBiocideAnimal repellantsPhosphorylationTumor transplantation
The use of eriocalyxin B in the manufacture of medicaments for treating leukemia, wherein the leukemia include leukemia containing AML1-ETO fusion protein, acute promyelocytic leukemia, acute myelocytic leukemia and lymphocytic leukemia. Eriocalyxin B increases the level of peroxidate and influences the phosphorylation and degradation of IκBα to prevent NF-κB from getting into the nucleus in order to inhibition NF-κB pathway. Eriocalyxin B can decrease the phosphorylation of ERI1 / 2 and inhibit MAPK pathway. Eriocalyxin B can extend the life span of mice and decrease tumor volume in mice model of C57 leukemia and tumor transplantation.
Owner:RUIJIN HOSPITAL ATTACHED TO SHANGHAI NO 2 MEDICALUNIV +1
Anti-melanoma compound and application thereof
InactiveCN106366080AInhibition of survivalGrowth inhibitionOrganic active ingredientsOrganic chemistryMelanomaNatural product
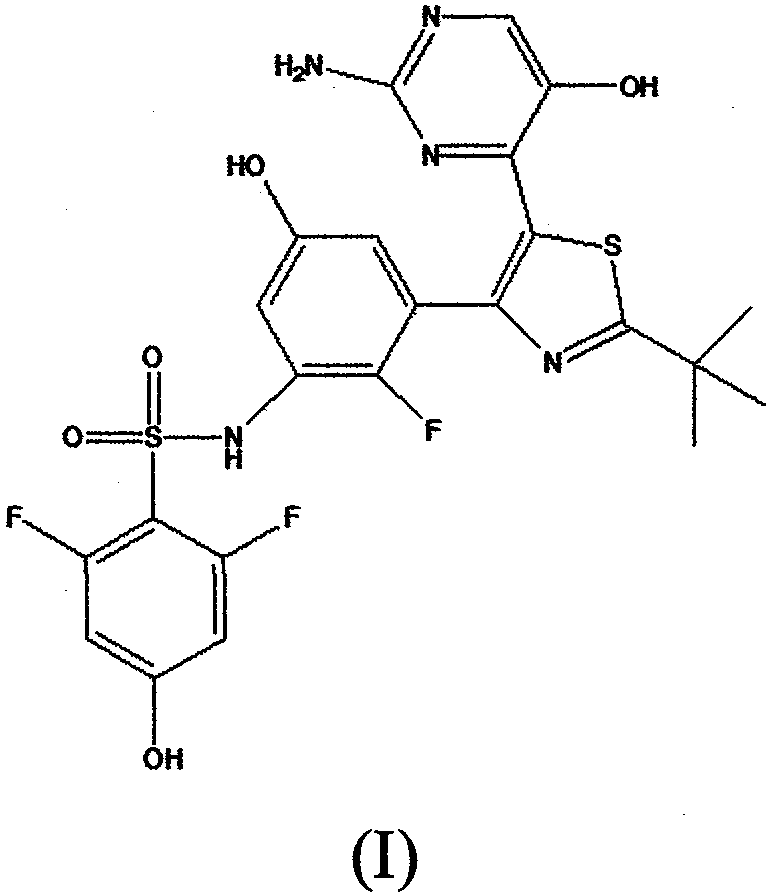
The invention discloses an anti-melanoma compound. The compound is named as N-(3-(5-(2-amino-5-hydroxypyrimidin-4-yl)-2-tert-butylthiazol-4-yl)-2-fluoro-5-hydroxyphenyl)-2,6-difluoro-4-hydroxybenzenesulfonamide, and the structural formula of the compound is represented by formula I. The compound is extracted from a natural product, and is evaluated by using melanoma cell strains and immunodeficiency SCID mouse tumor transplantation models. In vivo and in vitro tests show that the compound can substantially inhibit growth of all melanoma cell strains, and also can effectively inhibit growth of human derived melanoma cells in mouse bodies. The compound has a safe, highly-efficient and ideal anticancer effect, can be processed to possibly develop novel anti-melanoma treatment medicines, and has important development and application values.
Owner:CHONGQING UNIV
Drug composition for treating or inhibiting solid tumor and drug box containing composition
InactiveCN112294964AHigh antitumor efficacy in solid tumorsBroad solid tumor indication spectrumCancer antigen ingredientsImmunological disordersTumor transplantationPharmaceutical drug
The invention relates to a drug composition for treating or inhibiting solid tumor, a drug box containing the composition and an anti-solid tumor method. The drug composition includes a graft outsidetumor and an anti-transplantation drug for genetically identical tumor transplantation.
Owner:CHENGDU KUACHANGAOPU MEDICAL TECH CO LTD
Novel mouse in-situ pleural mesothelioma model and establishment method thereof
InactiveCN113599010ARestore invasive abilityRestore the effect of tumor metastasisAnimal fetteringSurgical veterinaryAnterior axillaryTumor transplantation
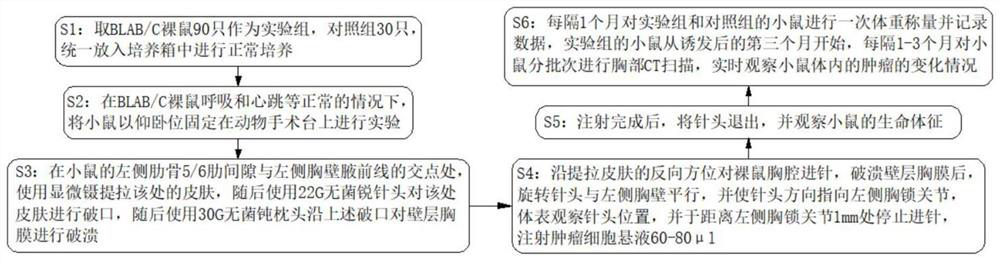
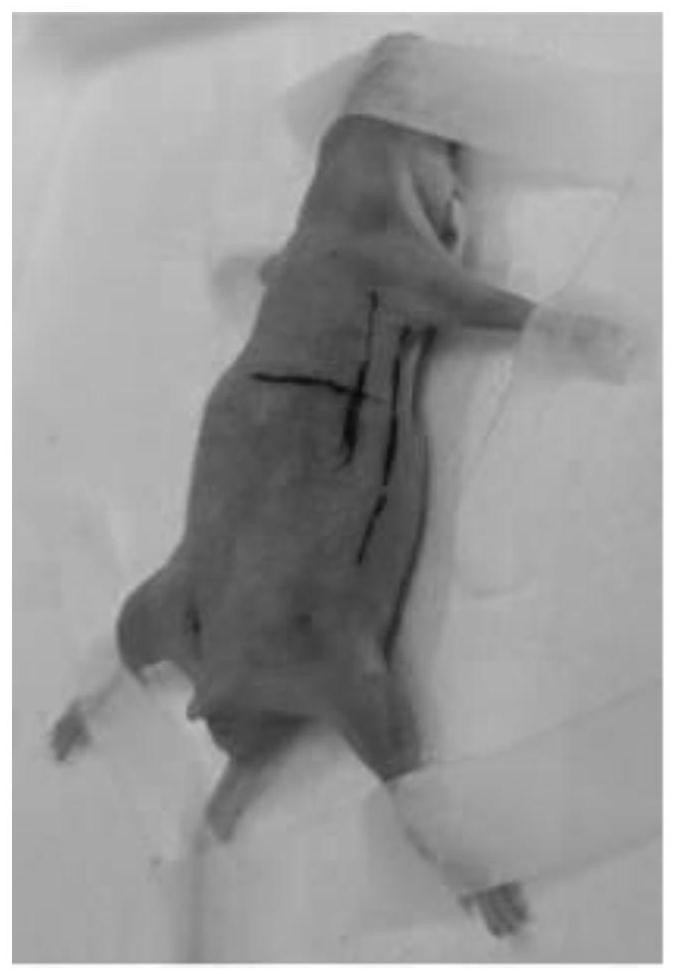
The invention discloses a novel mouse in-situ pleural mesothelioma model and an establishment method thereof. The establishment method comprises the following steps of 1, taking 90 BLAB / C nude mice as an experimental group and 30 control groups, and uniformly putting the experimental group and the control groups into an incubator for normal culture; 2, under the condition that BLAB / C nude mice breathe, heartbeat and the like are normal, anesthetizing the mice and fixing the mice on an animal operating table in a supine position for experiment; and 3, at the intersection point of the left rib 5 / 6 intercostal space and the left chest wall anterior axillary line of the mouse, using microscopic forceps to lift and pull the skin at the intersection point, then using a 22G sterile sharp needle head to break the skin at the intersection point, then using a 30G sterile blunt pillow to break the wall layer pleura along the broken opening. The tumor transplantation method provided by the invention is applicable to orthotopic transplantation of pleural tumors. The growth mode, the invasion ability and the tumor metastasis effect of the pleural tumor can be restored to the maximum extent, and a good mouse model is constructed for studying the pleural tumor.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
Application of L858R+V834L gene mutation primary tumor transplantation tumor model for human non-small cell lung cancer
The invention discloses application of an L858R+V834L gene mutation primary tumor transplantation tumor model for a human non-small cell lung cancer. A transplantation tumor generated by the L858R+V834L gene mutation primary tumor transplantation tumor model for the human non-small cell lung cancer can be restrained by Iressa; the L858R+V834L gene mutation primary tumor transplantation tumor model provided by the invention can be used for screening a targeted drug for treating an EGRF (estimated glomerular filtration rate) mutation positive patient suffered from the non-small cell lung cancer; and an experimental technical platform is provided for the deep exploration of the action of epidermal growth factor receptor (EGFR) mutation generated in the non-small cell lung cancer.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20130028888A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAutoimmune conditionAutoimmune disease
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:AERES BIOMEDICAL
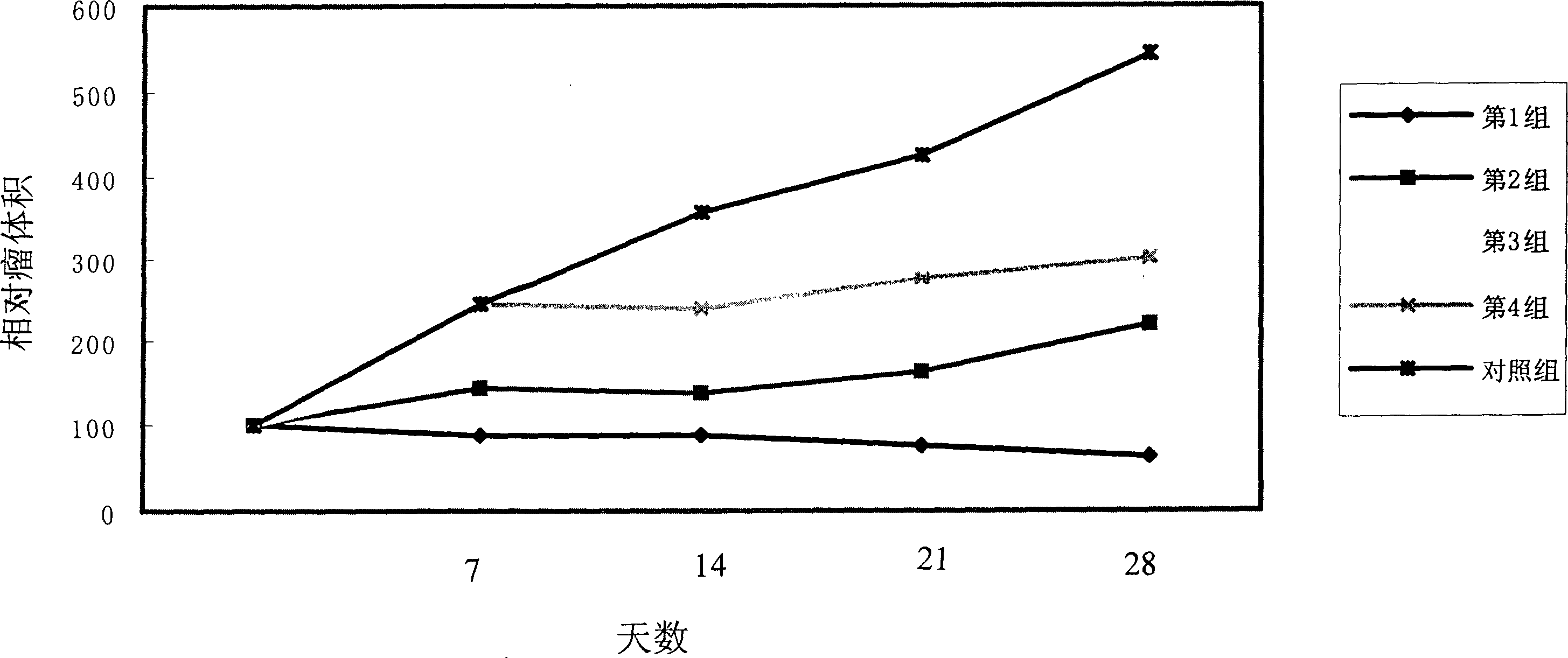
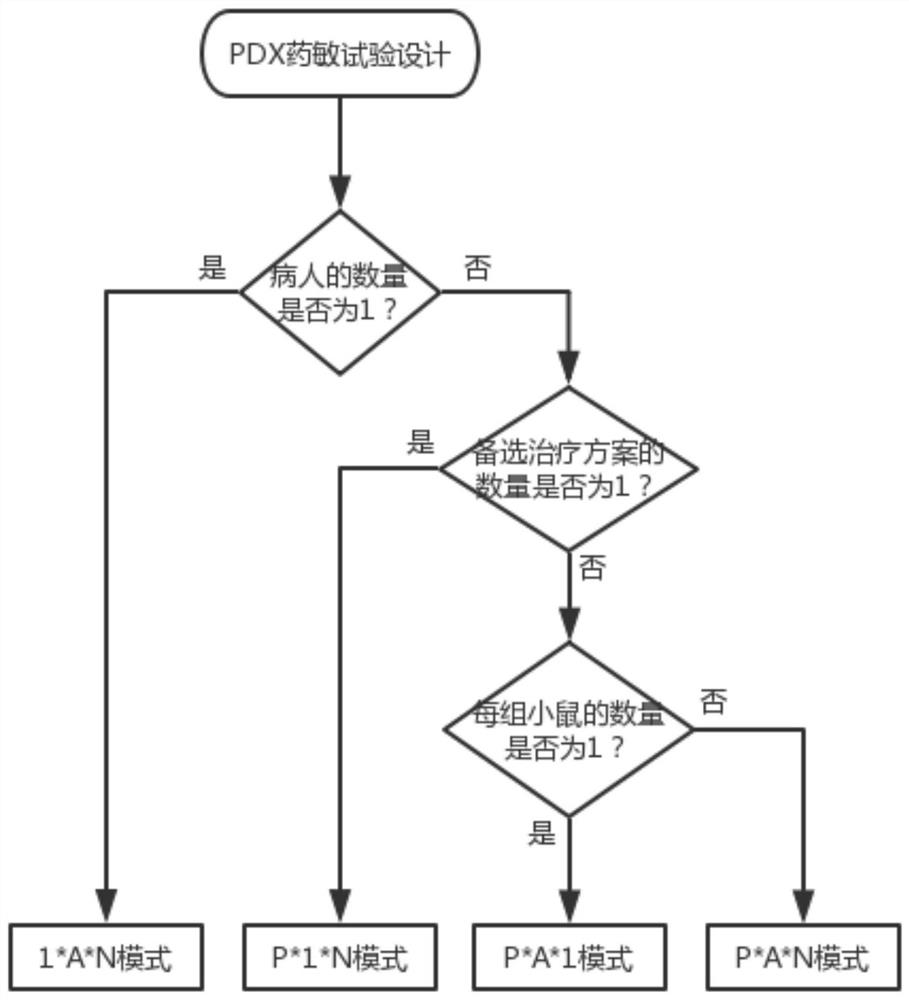
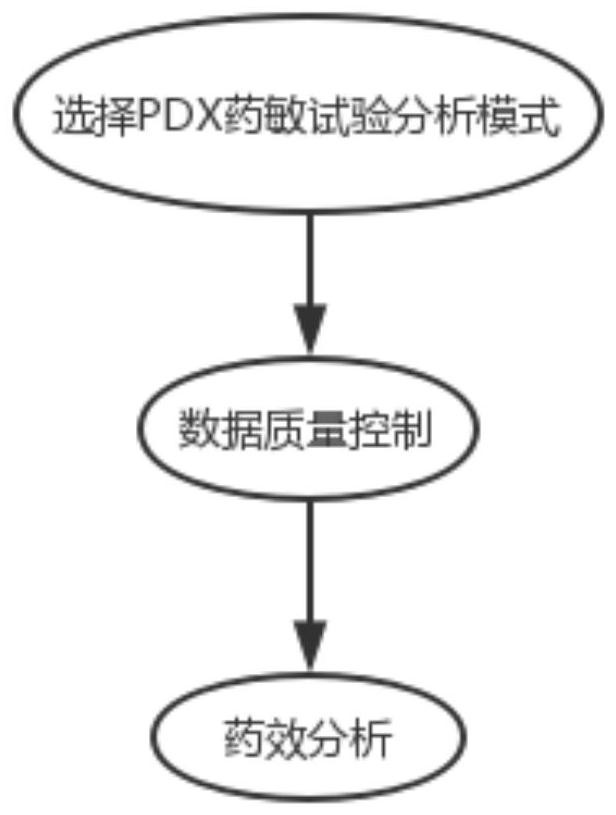
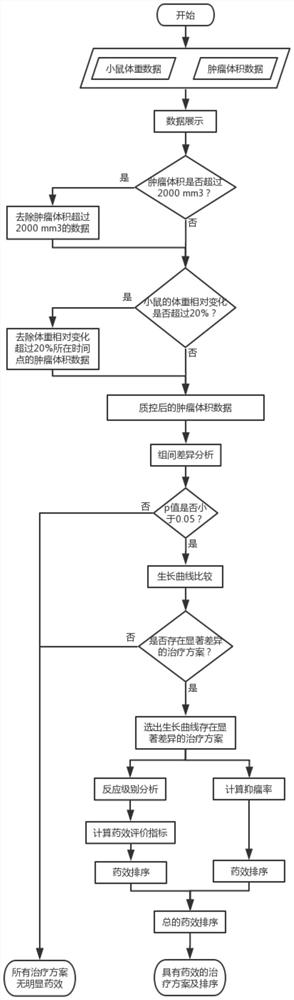
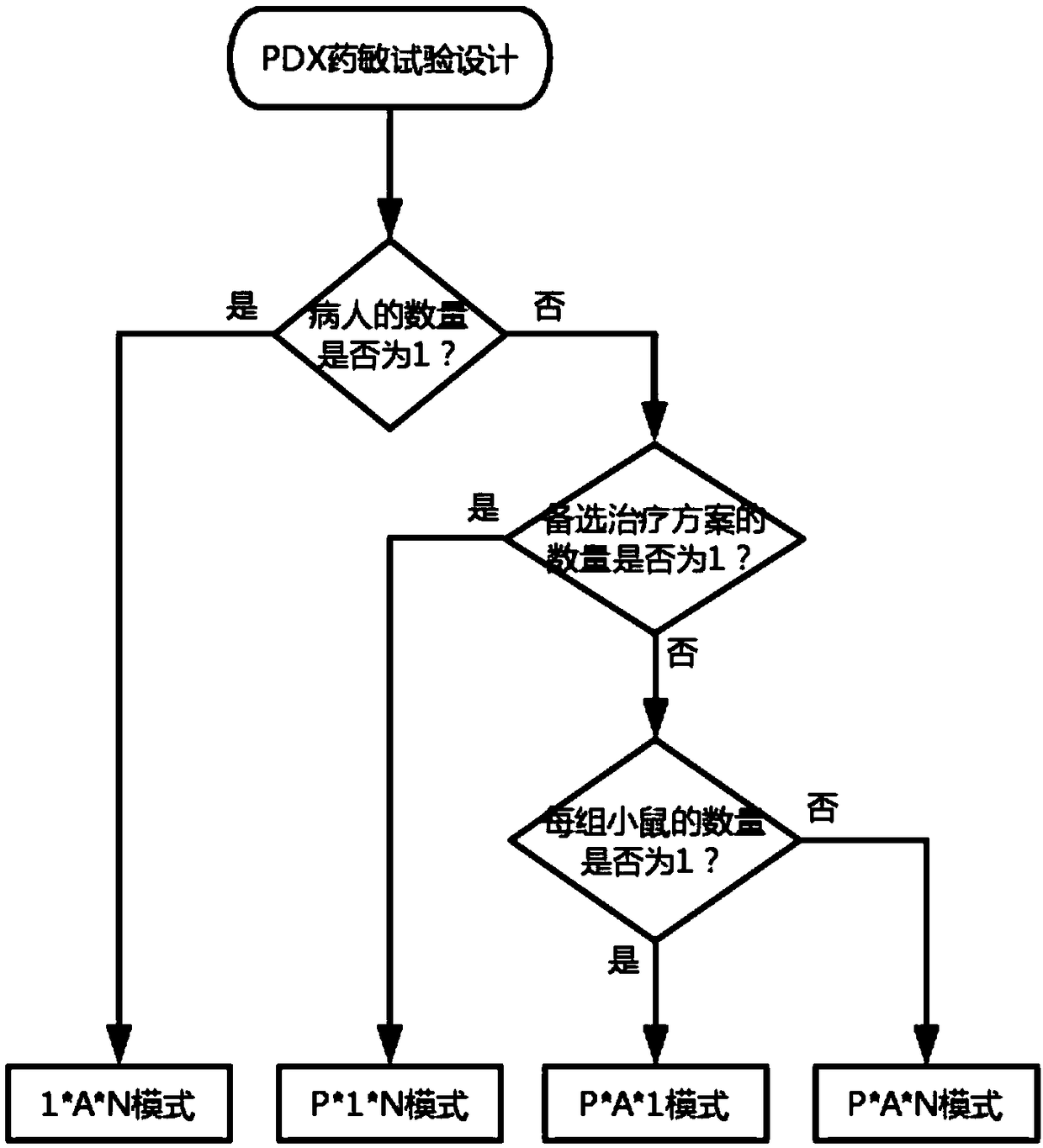
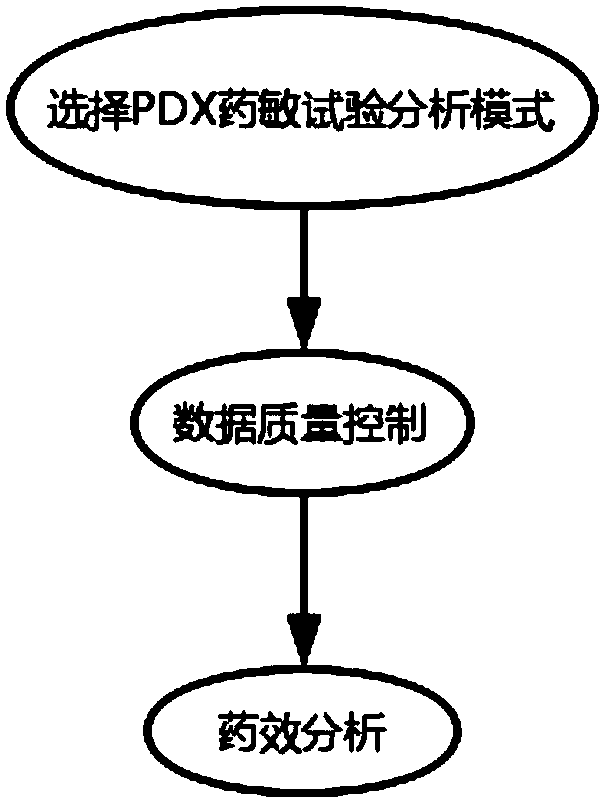
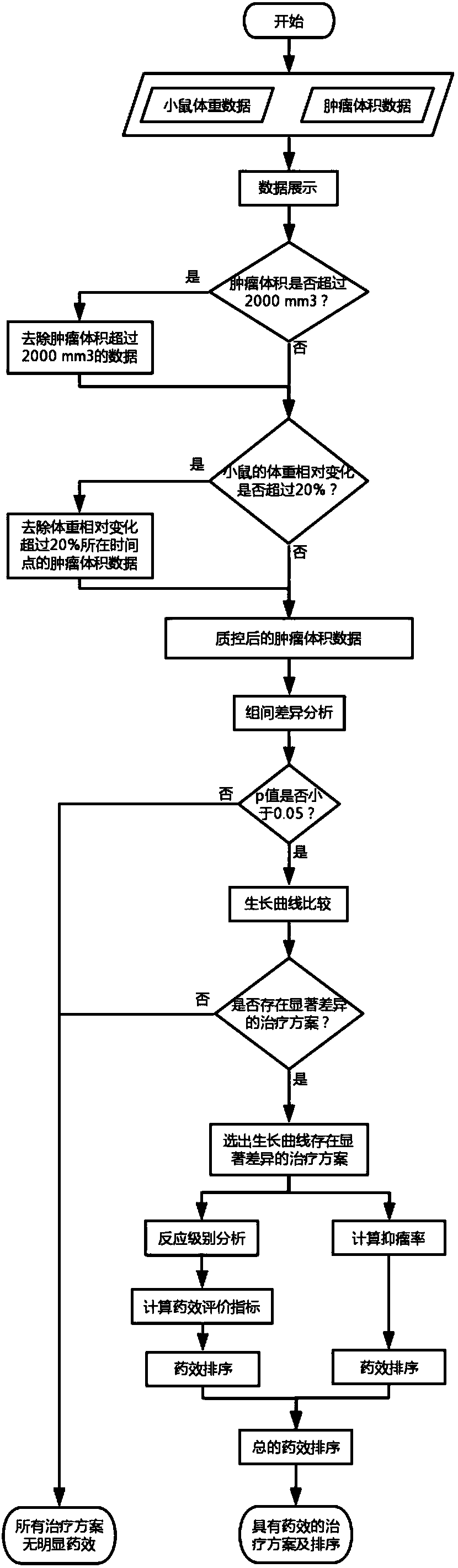
Drug susceptibility analysis method, analysis system and application for non-diagnostic and therapeutic purposes of tumor transplantation model
The invention discloses a drug sensitivity analysis method suitable for non-diagnostic and therapeutic purposes of tumor transplantation models, comprising the steps of: selecting a PDX drug sensitivity test response mode; data quality control; and statistically analyzing whether there is a significant tumor volume between each experimental arm difference; compare the tumor volume growth curves between each experimental arm and the blank control group; determine the drug sensitivity level of each experimental mouse, and calculate the drug efficacy evaluation index disease control rate of each experimental arm; calculate each The tumor inhibition rate of the experimental arm; the drug efficacy of different experimental arms was sorted according to the disease control rate and tumor inhibition rate. The method of the invention includes the selection of experimental modes, data quality control, data analysis and result display, and provides a multi-level and complete solution for PDX drug sensitivity analysis.
Owner:上海朴岱生物科技合伙企业(有限合伙)
A fusion protein containing tumor necrosis factor-related apoptosis-inducing ligand, its preparation method, and nanoparticles self-assembled by the protein
ActiveCN106519040BIncreased apoptotic activityAchieving soluble expressionBacteriaPeptide/protein ingredientsEscherichia coliIntraperitoneal route
The invention belongs to the field of bio-pharmaceuticals and particularly relates to expression, purification and self-assembly of TRAIL variant proteins. A recombinant protein is expressed by plasmids comprising the TRAIL fusion proteins in escherichia coli, and the recombinant protein with high activity is produced. The recombinant proteins adopt a centrifugal mode for simple and rapid purification. The purified proteins are self-assembled into particles being 200 nanometers under the physiological conditions. After intravenous injection to a tumor transplantation mouse, the recombinant protein is effectively gathered in the tumor location of the mouse. The recombinant protein is injected into the tumor transplantation mouse in an intraperitoneal injection mode, and tumor growth is effectively suppressed. The recombinant protein subjected to simple process purification is used for oncotherapy.
Owner:NANJING UNIV
Application of a kind of mir-100 inhibitor in preparation of medicine for treating breast cancer
ActiveCN103893780BGrowth rate inhibitionInduce apoptosisGenetic material ingredientsAntineoplastic agentsAntisense nucleic acidApoptosis
The invention discloses application of a miR-100 inhibitor in preparation of a medicine for treating a breast cancer. The miR-100 inhibitor is a miR-100 antisense nucleic acid chain, and the sequence is 5'-TTCGGATCTACGGGTT-3'. The miR-100 inhibitor disclosed by the invention can induce apoptosis of tumor cells, growth of tumors in tumor transplanted mice can be effectively induced, the sensitivity of breast cancer cells on chemotherapy medicines is increased, the effect of chemotherapy is improved, and the miR-100 is a latent new target for the medicine for treating the breast cancer, and is a new path for medical therapy.
Owner:ZHEJIANG UNIV +1
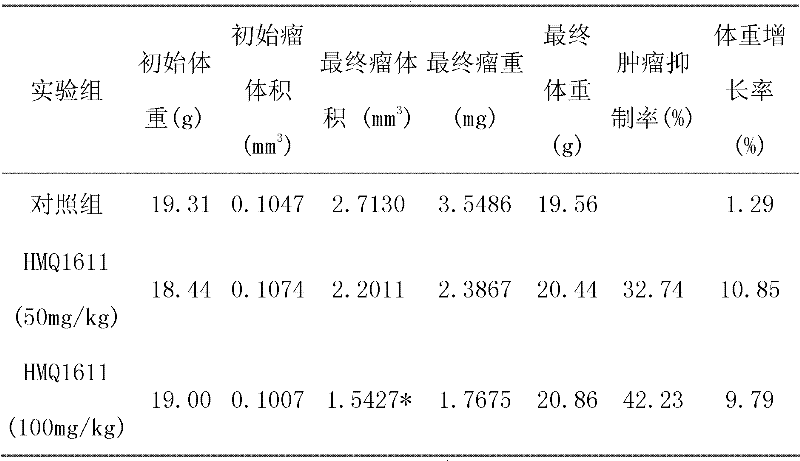
Application of thaspine derivative HMQ1611 in inhibition of mammary cancer
InactiveCN102349890AValidation of inhibitionVerify impactOrganic active ingredientsSexual disorderTumor transplantationEstrogen receptor
The invention relates to application of thaspine derivative HMQ1611 in inhibition of mammary cancer. In an integral level, a mammary cancer ZR-75-30 tumor transplantation tumor model is built on the basis of a nude mouse transplantation tumor model, the inhibition effect of the thaspine derivative HMQ1611 on mammary cancer is researched, and then the mammary cancer resistance passageway of the thaspine derivative HMQ1611 is also researched, thus the inhibition effect of the thaspine derivative HMQ1611 on ER (estrogen receptor)-dependent mammary cancer is verified, and the influence of the thaspine derivative HMQ1611 on ER passageway of mammary cancer cells is also verified, thereby showing that the thaspine derivative HMQ1611 can truly inhibit mammary cancer.
Owner:XI AN JIAOTONG UNIV
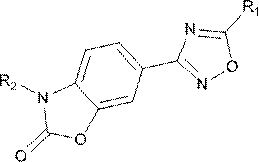
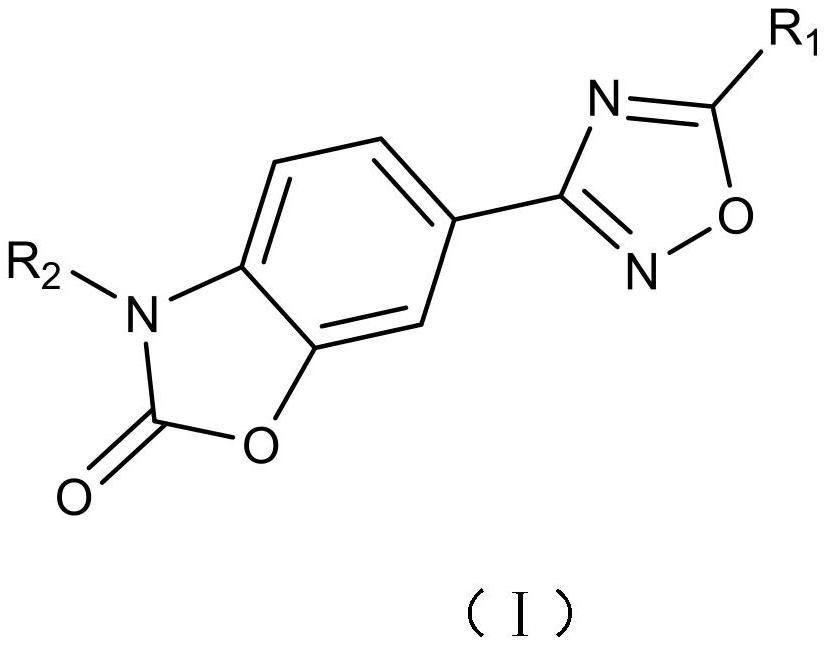
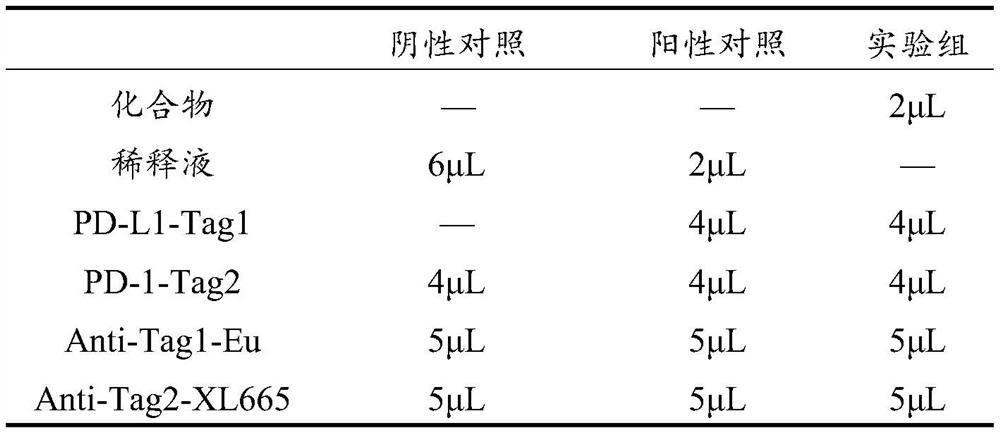
Small molecule compounds inhibiting PD-1/PD-L1 and their uses
ActiveCN108774219BHas antitumor activityOrganic active ingredientsOrganic chemistryChemical compoundTumor transplantation
The invention discloses the use of a compound or a pharmaceutically acceptable salt, ester or solvate thereof in the preparation of a PD-1 / PD-L1 small molecule inhibitor drug. In the present invention, candidate molecules with potential activity in the compound library are screened out through computer simulation, and homogeneous time-resolved fluorescence technology is used to perform high-throughput screening on the binding of candidate molecules to antagonize PD-1 / PD-L1, and select those with better activity. The compound was subjected to a mouse melanoma B16-F10 tumor transplantation experiment, and it was determined that the compound screened by the present invention has anti-tumor activity.
Owner:CHINA PHARM UNIV
Drug sensitivity reaction analysis method of patient-derived xenograft, analysis system and application thereof
The invention discloses a drug sensitivity reaction analysis method of a patient-derived xenograft. The analysis method comprises the following steps: selecting a PDX (Patient-Derived Xenograft) drugsensitivity testing reaction mode; carrying out data quality control; counting and analyzing whether gross tumor volumes of different experiment arms have remarkable differences or not; comparing whether the experiment arms have differences with gross tumor volume growth curves of blank control groups; determining the drug sensitivity reaction grade of each testing mouse, and calculating the drugeffect evaluation index disease control rate of each experiment arm; calculating the anti-tumor rate of each experiment arm; and according to the disease control rate and the anti-tumor rate, sequencing drug effects of the different experiment arms. The method disclosed by the invention comprises steps of experiment mode selection, data quality control, data analysis and result demonstration, anda multi-layer and complete solving scheme is provided for PDX drug sensitivity analysis.
Owner:上海朴岱生物科技合伙企业(有限合伙)
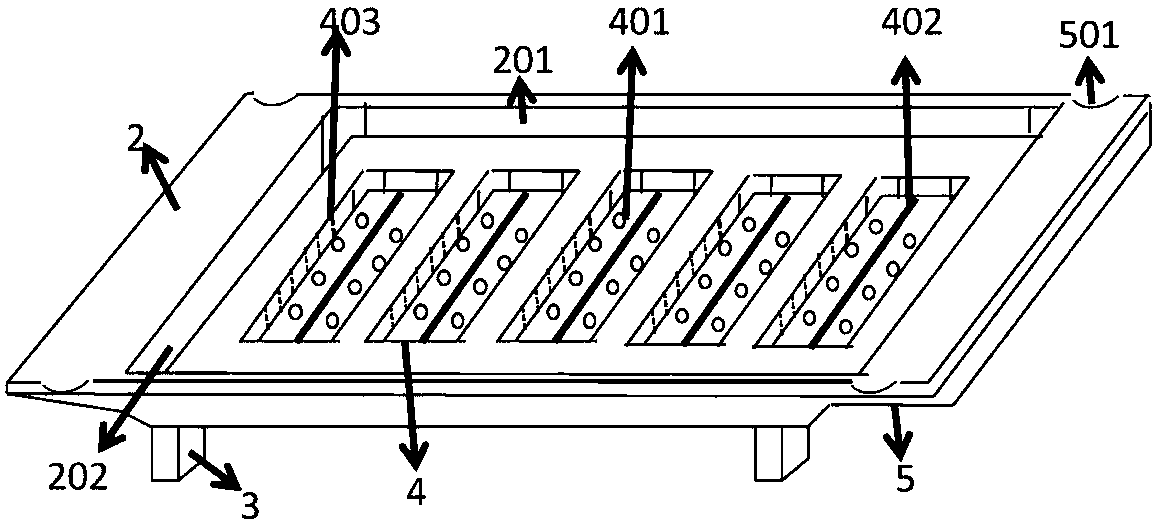
Special device for tumor transplantation of zebrafish larvae, and working method of special device
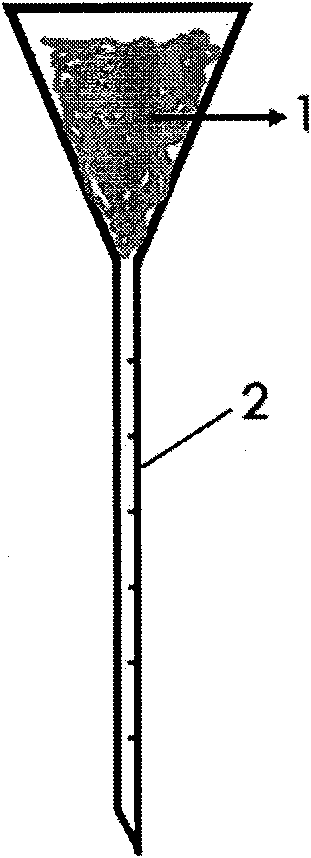
The invention relates to a special device for tumor transplantation of zebrafish larvae, and a working method of the special device. The special device comprises a box body and a transplantation body,the box body and the transplantation body are each of a rectangular concave body with baffles being arranged around, and the transplantation body and the box body are combined into a whole when the transplantation body is inserted into the box body. Four cuboid vertical columns are arranged at the four corners of the lower bottom face of the transplantation body correspondingly, and a plurality of grooves are formed in the inner bottom face of the transplantation body; and a plurality of drainage holes and a scale are arranged on the inner bottom face of each groove, and a plurality of sets of scale marks are arranged on the left inner side wall and the right inner side wall of each groove. According to the special device, the position of the larvae is fixed during tumor transplantation advantageously, and operation is convenient; and the dosage of transplanted tumor cells can also be quantified accurately. In addition, through the special device, the situation that the larvae are sucked mistakenly in the process of sucking excess liquid before tumor transplantation, and consequently, the larvae are damaged can further be effectively avoided; the transplantation time is saved advantageously, and the situation that the operation time is excessively long, consequently, the larvae die due to water shortage is avoided; and the culture device is ingenious in design concept, convenient to use, obvious in effect and good in effect, experiment accuracy is ensured, and the working efficiency is improved.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
Application of Japanese ginseng polysaccharide to preparation of medicine for improving immunologic function of patients suffering from liver cancer
InactiveCN107929307AGood workmanshipImprove controllabilityOrganic active ingredientsImmunological disordersT-cell apoptosisCD8
The invention belongs to the technical field of medicine application of plant polysaccharide and discloses the application of Japanese ginseng polysaccharide to the preparation of a medicine for improving immunologic function of patients suffering from liver cancer. The invention discovers for the first time that the Japanese ginseng polysaccharide can obviously inhibit in-vivo growth of H22 livercancer cells under a certain dosage, and the Japanese ginseng polysaccharide obviously increases the proportion of CD8+T and NK cells by increasing thymus / spleen index of the organism and spleen cellproliferation and enhances the cytotoxic activity on the liver cancer H22 tumor cells. The Japanese ginseng polysaccharide can stimulate immune response of the mouse organism to exert the anti-tumoreffect, and the toxicity on the tumor-bearing mouse is not detected. The related action mechanism comprises the followings: the Japanese ginseng polysaccharide can improve the ability of tumor transplantation induced CD4+T cell apoptosis; the immunostimulation factor level in the tumor-bearing mouse is increased and the immunosuppression factor level is reduced; the tumor-bearing mouse CD4+T cellsare promoted to be differentiated into Th1 to take part in anti-tumor immunostimulation activity; and the immunostimulation activity is generated by resisting inflammation and adjusting polarizationof TAMs, so that the anti-tumor effect is achieved.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Pharmaceutical composition for treating kidney cancer and application thereof
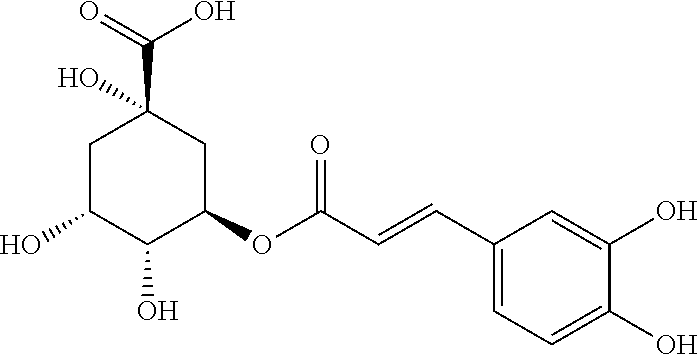
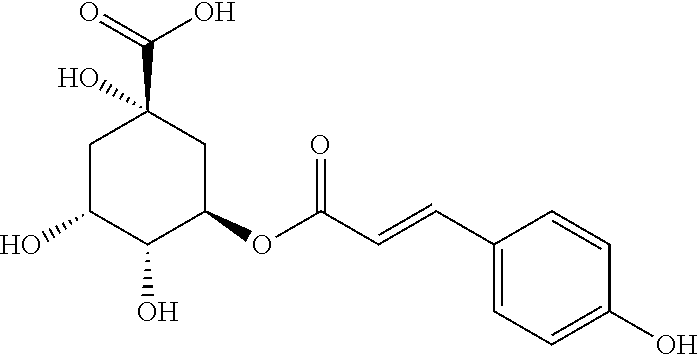
PendingUS20210330626A1Obvious effectConvenient for clinical operationAntineoplastic agentsHeterocyclic compound active ingredientsChlorogenic acidCancer cell
A pharmaceutical composition contains chlorogenic acid and coumaroyl quinic acid. The combined use of chlorogenic acid and coumaroyl quinic acid at a certain ratio can generate a synergistic enhancing effect to effectively inhibit the growth of human kidney cancer cell strain A479, rat kidney cell strain RuCa, and rat transplanted tumor RuCa, providing a novel choice for clinical treatment of kidney cancer and having good market application prospects.
Owner:SICHUAN JIUZHANG BIO TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com