3-methoxylflavonoid compound, preparation method and application thereof
A technology of methoxyflavone and trimethoxyflavone, applied in organic chemistry, drug combination, pharmaceutical formula, etc., can solve the problem of accumulation of chemotherapy drugs and achieve the effect of inhibiting tumor growth, weight loss and good growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Preparation of compound A 5,7-dihydroxy-8-(3,3-dimethylallyl)-3,3',4'-trimethoxyflavone
[0054] (1) 10g (55mmol) 3,4-dimethoxybenzoic acid and 2.2ml (28.4mmol) methanesulfonyl chloride were reacted at room temperature for 2 hours to obtain 8.47g 3,4-dimethoxybenzoic anhydride, The yield was 89%.
[0055] (2) 1.6g (8.1mmol) 2', 4', 6'-trihydroxyl-2-methoxyacetophenone and 8.4g (21.0mmol) 3,4-dimethoxybenzoic anhydride in 100ml Et 3 Reacted at 40°C in N for 4 hours, and then reacted at 40°C in 100ml of 5% KOH methanol solution for 2 hours to obtain 2.2g of 5,7-dihydroxy-3,3',4'-trimethoxyflavone crude product , yield 74%. In Hexane: Me 2 Recrystallized from a CO (50:50, V / V) mixture to obtain 0.85 g of pure 5,7-dihydroxy-3,3',4'-trimethoxyflavone.
[0056] (3) 0.34g (0.98mmol) 5,7-dihydroxy-3,3',4'-trimethoxyflavone, 0.25ml (1.97mmol) isoprene bromide and 0.4g anhydrous K 2 CO 3 (2.0 mmol) in 50 ml of acetone was reacted at room temperature for 5 hours. ...
Embodiment 2
[0062] Example 2 Compound B 5, the preparation of 7-dihydroxy-8-(3,3-dimethylallyl)-3,4'-dimethoxyflavone
[0063] (1) 10 g (65.7 mmol) of p-methoxybenzoic acid and 2.6 ml (33.6 mmol) of methanesulfonyl chloride were reacted at room temperature for 2 hours to obtain 6.13 g of p-methoxybenzoic anhydride, with a yield of 89%.
[0064] (2) 0.6g (3mmol) 2', 4', 6'-trihydroxy-2-methoxyacetophenone and 2.6g (9mmol) p-methoxybenzoic anhydride in 100ml Et 3 React at 40°C for 4 hours in N, and then react at 40°C for 2 hours in 100 ml of 5% KOH methanol solution. 0.763 g of ergonovine was obtained with a yield of 81%.
[0065] (3) 0.30g (0.95mmol) ergonovine, 0.2ml (1.73mmol) isoprenyl bromide and 0.4g anhydrous K 2 CO 3 (2.0 mmol) in 50 ml of acetone was reacted at room temperature for 5 hours. After filtration, the solid obtained after the filtrate was evaporated was recrystallized in methanol to obtain 0.33 g of 5-hydroxy-7-prenyloxy-3,4'-dimethoxyflavone with a yield of 91%.
...
Embodiment 3
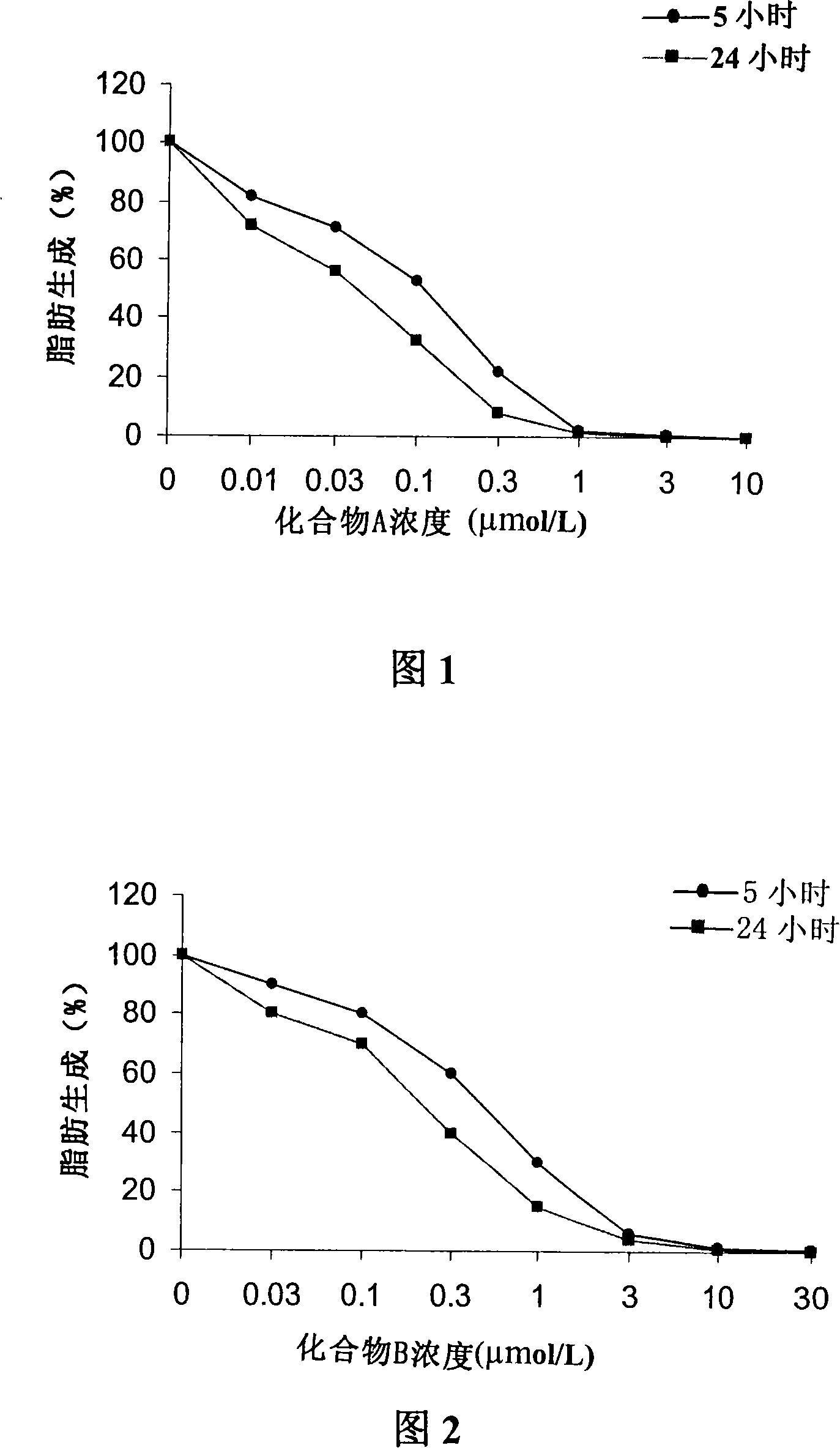
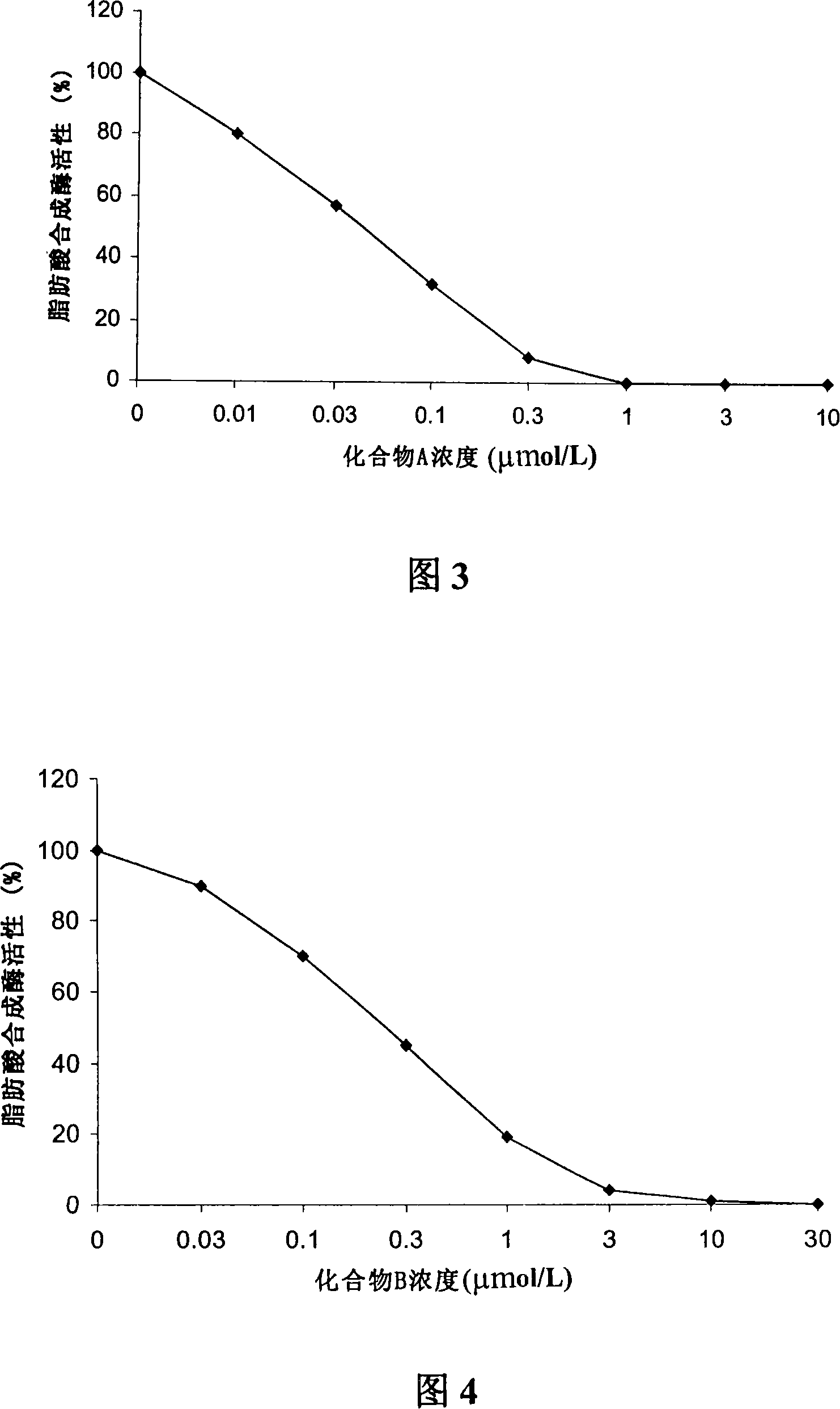
[0071] Embodiment 3 uses [2- 14 C] Acetate incorporation method to determine the inhibitory ability of compound A or compound B to LnCAP cell lipogenesis
[0072] Treat human prostate cancer cell LnCAP with different concentrations of compound A or compound B for 5 hours or 24 hours, add 2- 14 C-labeled acetate, incubate for 4 hours, collect culture medium and cells, centrifuge, and resuspend in 0.8ml PBS. Apply the Bligh Dyer method (Swinnen J.V. et al., Endocrinology, 1996, 137, 4468-4474.) to extract lipids, and apply the scintillation counting method to quantify [2- 14 C] Acetate, the results obtained were corrected for the protein content of the sample. Compound A or Compound B inhibits LnCAP lipogenesis-concentration curves of human prostate cancer cells as shown in Figure 1 and Figure 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com