A fusion protein containing tumor necrosis factor-related apoptosis-inducing ligand, its preparation method, and nanoparticles self-assembled by the protein
An apoptosis-inducing ligand and tumor necrosis factor technology, which is applied in the preparation method of peptides, chemical instruments and methods, and microbial-based methods, can solve problems such as difficulty in obtaining solubility, affecting anti-tumor effect, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
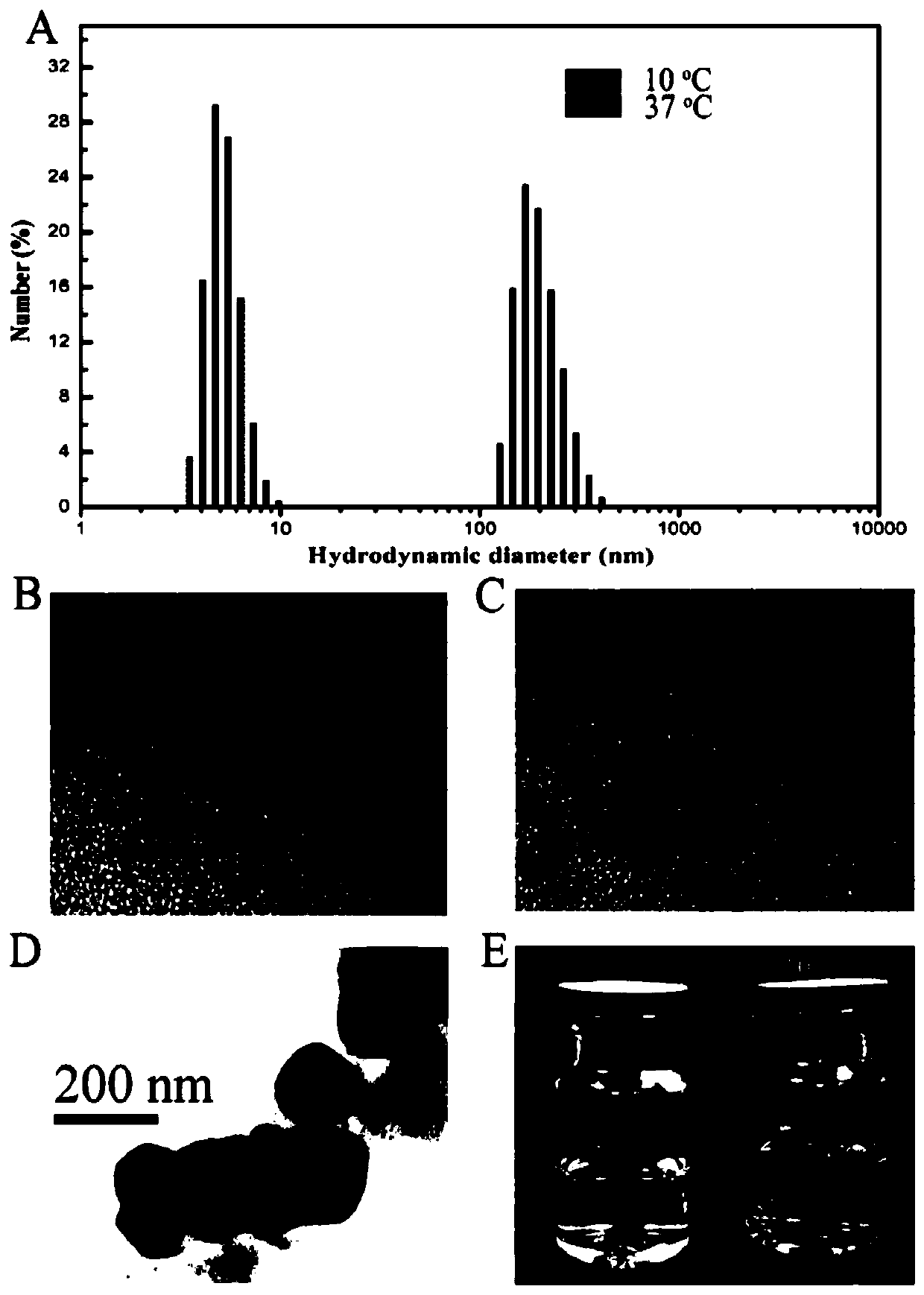
Image
Examples
Embodiment 1
[0027] Example 1 Construction of RGD-TRAIL-ELP plasmid
[0028] ELP[VH 4 -5] The gene sequence is: 5'-CCAC GGC GTGGGTGTTCCGGGCGTAGGTGTCCCAGGTCACGGCGTACCGGGCCACGGTGTTCCTGGTCACGGCGTGCCG GGC TGGC-3 (its amino acid sequence is VPG V G-VPG H G-VPG V G-VPG H G-VPG H G), synthesized by Nanjing GenScript Company, ELP[VH 4 -40] (8 ELP[VH 4 -5] sequence repeat) by ELP [VH 4 -5] extended by the RDL method; ELP[VH 4 -40] connected with the RGD-TRAIL gene fragment pET-23a through BamHI and HindIII. All target gene sequences were identified by sequencing (T7 and T7-ter). It was identified by sequencing that the RGD-TRAIL-ELP gene had been inserted into NdeI and HindIII in pET-23a.
Embodiment 2
[0029] Example 2 Expression, purification and identification of RGD-TRAIL-ELP
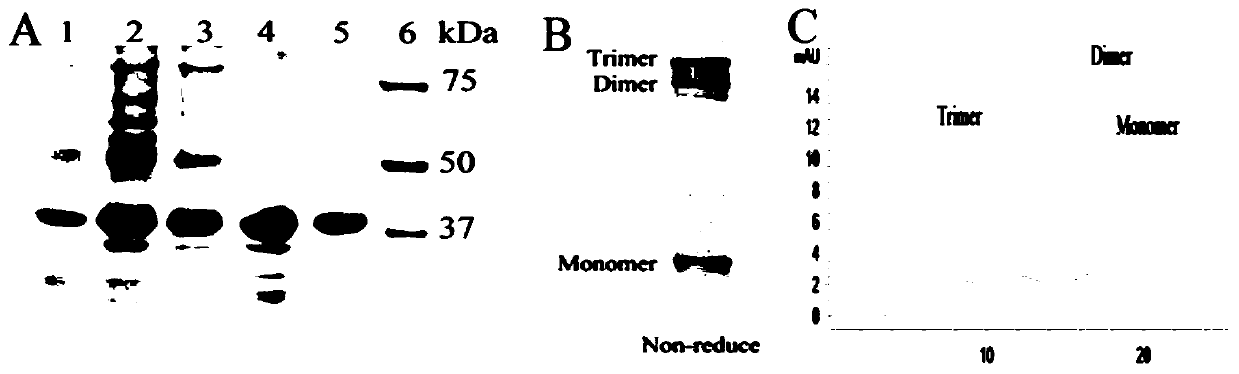
[0030]After the RGD-TRAIL-ELP plasmid was transferred into BL21(DE3), engineered bacteria were constructed and cultured in 4 liters of TB medium. When the OD value reached 0.6, induction was added with IPTG and induced overnight at 30°C. The bacteria were collected and used Ultrasonic breaker for cell disruption, bacterial disruption liquid centrifuged at 4 degrees at 12000g for 5 minutes to take the supernatant; heat the supernatant at 40 degrees, centrifuge at 40 degrees at 12000g for 5 minutes, take the precipitated part; put the precipitated part in pre-cooled PBS Dissolve the buffer solution, put it at 4°C for 15 minutes, centrifuge at 12000g at 4°C for 5 minutes to take the supernatant, then centrifuge at 4°C to take the supernatant and take the precipitate at 40°C for three cycles.
[0031] Use SDS-PAGE to identify the soluble expression of RGD-TRAIL-ELP in E.coli and analyze the protein pur...
Embodiment 3
[0033] Example 3 Detection of apoptosis-inducing effect of fusion protein
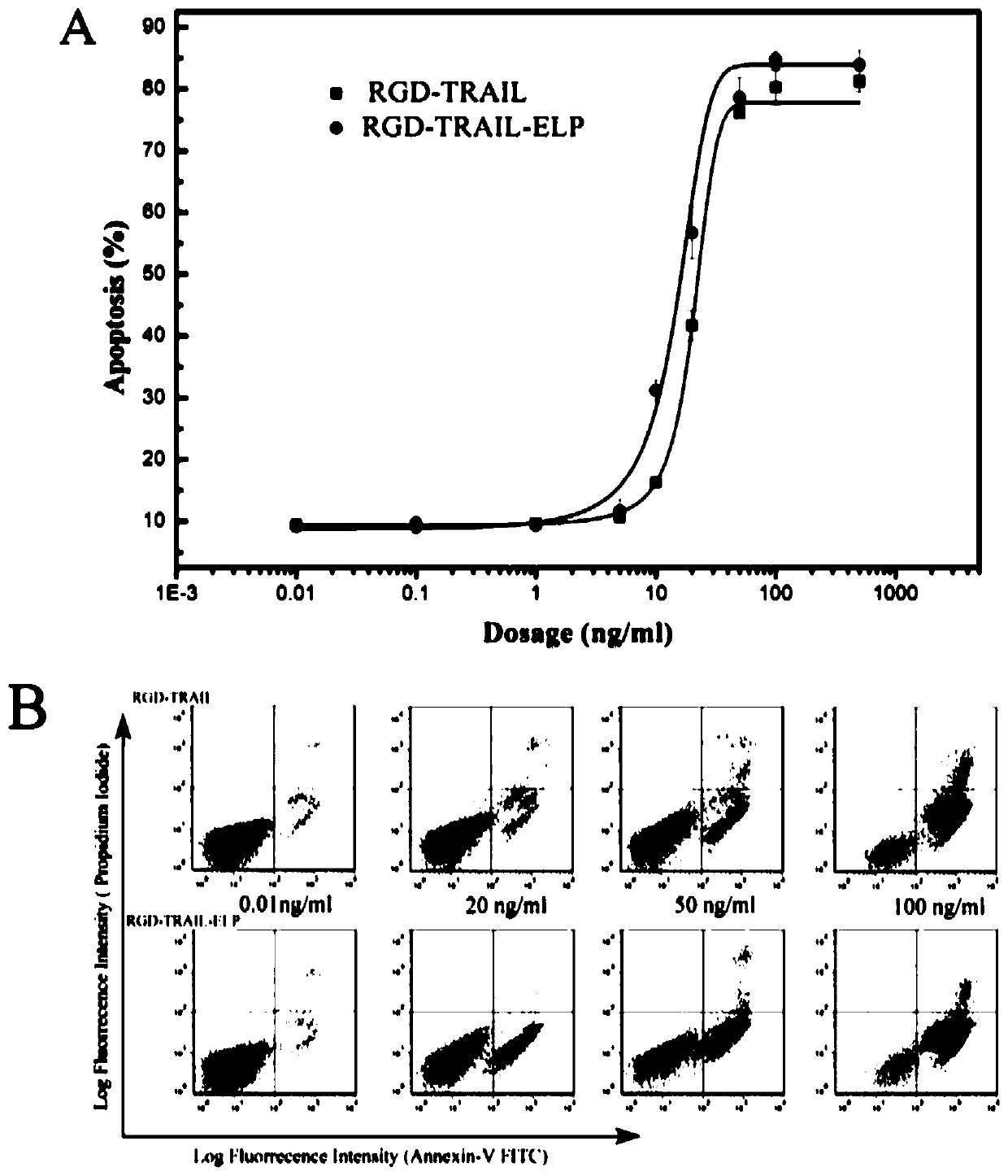
[0034] Human colon cancer cells (Human COLO rectal carcinoma cells, COLO205 cells) were used to detect the activity of the purified RGD-TRAIL-ELP. COLO205 was cultured in RPMI 1640 containing 10% bovine serum. cells (10 5 ) after a series of concentration gradients of RGD-TRAIL or RGD-TRAIL-ELP induction treatment, digested with trypsin, aspirated from the culture well, washed twice with PBS, centrifuged at 300g for 5 minutes, discarded the supernatant, and then used 300μL binding buffer The solution was resuspended, and Annexin V-FITC with a final concentration of 2 μg / mL was added to incubate at room temperature. After 10 minutes, the cells were transferred to flow tubes. Cytometry analysis.
[0035] The result is attached image 3 As shown, the half dose (EC50) of RGD-TRAIL and RGD-TRAIL-ELP is 1 and 0.35nM (20.57 and 13.61ng / ml), respectively, so the apoptosis-inducing activity of RGD-TRAIL-ELP...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com