Soluble aquaporin AqpZ fusion vector and method for constructing same
An aquaporin, soluble technology, applied in the biological field, can solve problems such as uncertainty and achieve the effect of eliminating influence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
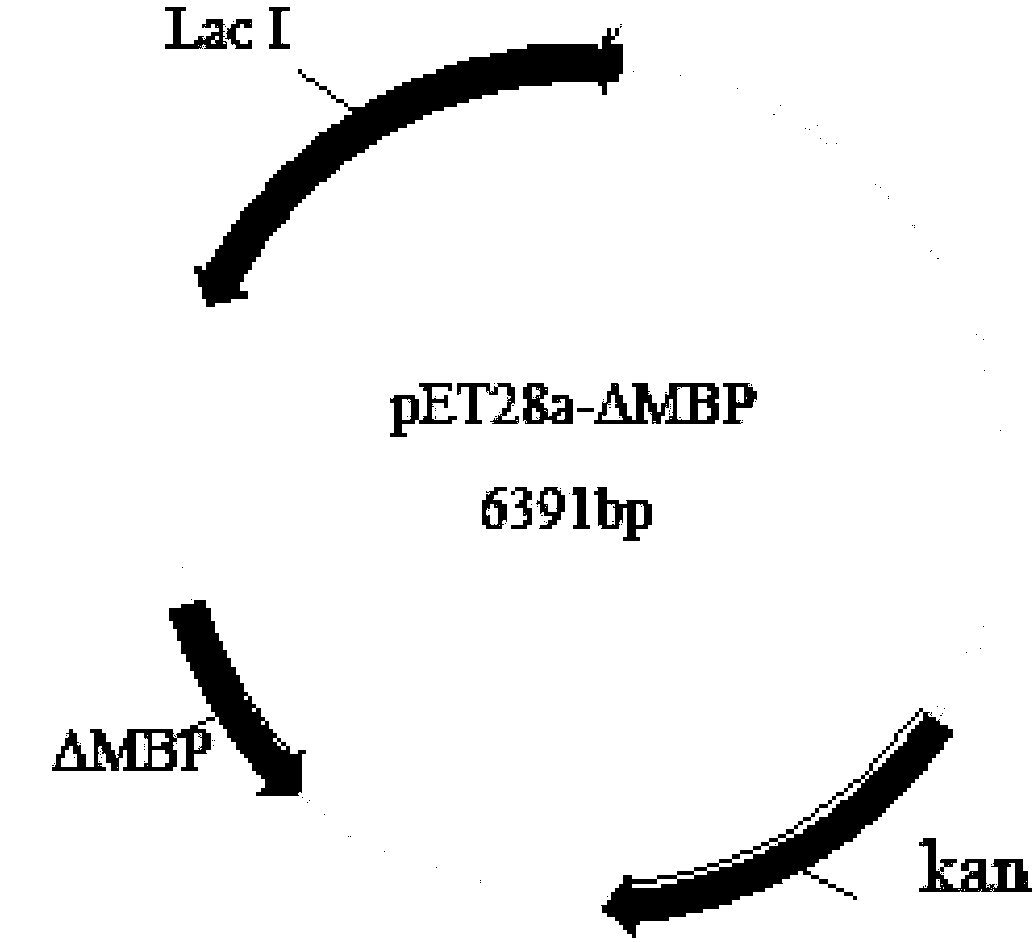
[0034] 1. A construction method of the maltose-binding protein carrier pET28a-ΔMBP without the N-terminal signal peptide:
[0035] a) Design a pair of primers based on the pMBP-p plasmid purchased from Wuhan Miaoling Biotechnology. The oligonucleotide sequence upstream of the primer is shown in SEQ ID No.1, and the oligonucleotide sequence downstream of the primer is shown in SEQ ID No.2. Using the pMBP-p plasmid as a template, the cDNA of the maltose-binding protein with the N-terminal signal peptide removed was obtained through PCR reaction. The PCR reaction system configuration is shown in Table 1.
[0036] Table 1 PCR reaction system preparation table
[0037]
[0038]
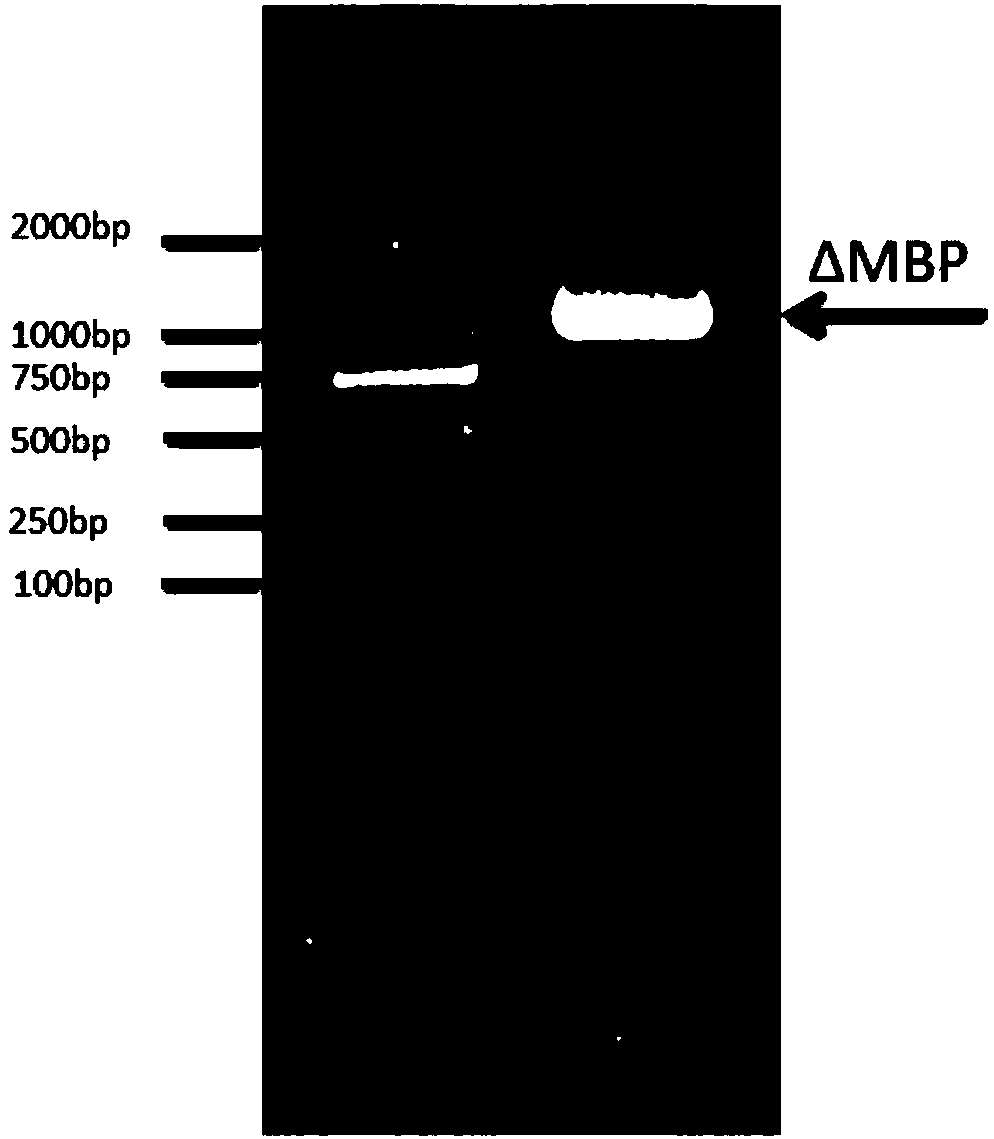
[0039] PCR results such as figure 1 shown. The first lane is Marker, the second lane is the target fragment ΔMBPcDNA, and the length of the target fragment is 1032bp.
[0040] b) Recover the maltose binding protein cDNA that removes the N-terminal signal peptide, use NcoI and NdeI to double dige...
Embodiment 2
[0056] Example 2: Expression method of a soluble aquaporin AqpZ vector
[0057] Transform the correctly identified pET28a-ΔMBP-AqpZ-ApoAI*-His plasmid into E. coli expression host BL21(DE) to obtain a single colony of stable transformation; inoculate a single colony into LB liquid medium containing 50 μg / ml kanamycin In 200 rpm, 37 ° C overnight culture, to obtain overnight bacteria. Inoculate overnight bacteria into TB medium at an inoculation ratio of 1:200, and culture to OD at 230 rpm at 30°C 600 After reaching 0.8-1.0, add IPTG (isopropylthiogalactopyranoside) with a final concentration of 0.1 mM, and then culture at 16°C for 18 hours. The cells were collected by centrifugation at 4000 rpm for 15 minutes. The bacteria were resuspended in PBS buffer, and then centrifuged at 4000 rpm for 15 minutes to obtain soluble fusion expression of aquaporin ΔMBP-AqpZ-ApoAI*-His bacteria.
Embodiment 3
[0058] Example 3: Verification of a soluble aquaporin AqpZ carrier
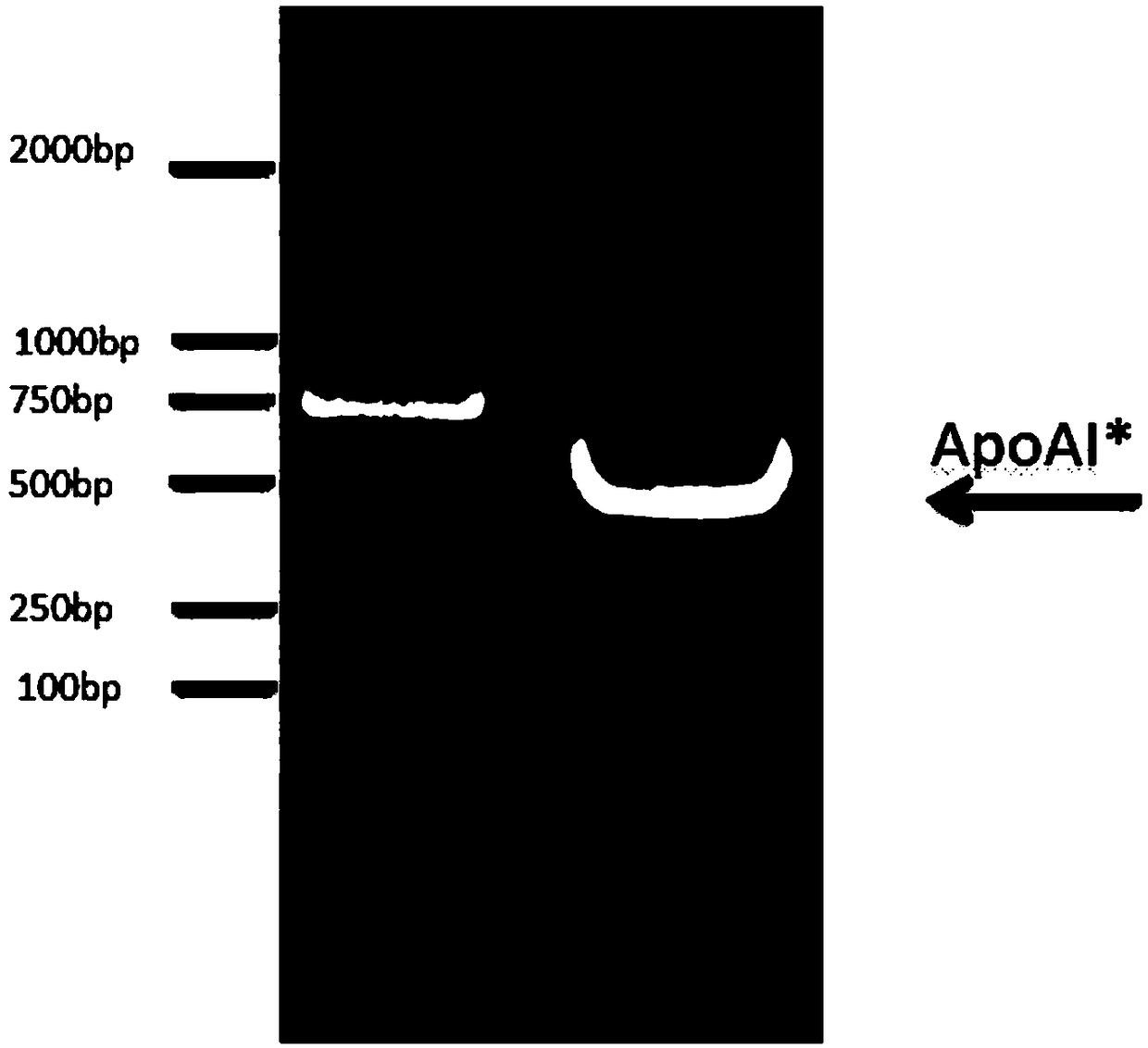
[0059] Step 1, sonicate the fusion protein ΔMBP-AqpZ-ApoAI*-His cells obtained in Example 2. The ultrasonic time is 15 minutes, the ultrasonic is 2 seconds, the interval is 4 seconds, and the ultrasonic power is 60W. Finally, centrifuge the sonicated bacteria at 10,000g at 4°C for 45 minutes, collect the supernatant to obtain the whole protein fraction; centrifuge the supernatant at 100,000g at 4°C for 2 hours, collect the supernatant to obtain the soluble fraction point.
[0060] Step 2: Collect the whole protein fraction and soluble fraction of the fusion protein, take 10 μL sample, add 10 μL 2*loading buffer, incubate at 75°C for 10 minutes, after cooling, centrifuge at 12,000g for 5 minutes, and apply 15 μL of sample; first Run glue at 90 volts for 30 minutes, then change to 150 volts and run glue for 50 minutes.
[0061] Step 3, use nitrocellulose membrane for wet transfer, the transfer condition is c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com