Borrelia garinii OspA protein C-terminal peptide fragment and application thereof
A technology of Borrelia garzii and its C-terminus, which is applied in the field of immunology, can solve problems such as unseen related researches, and achieve good immune protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
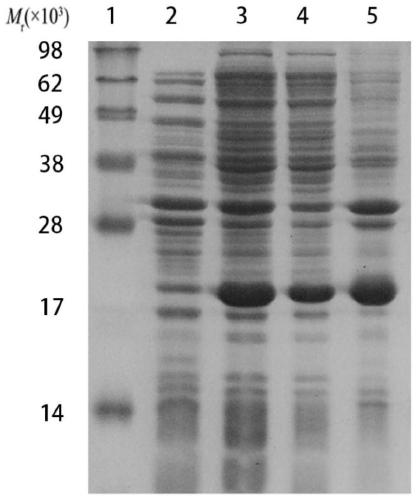
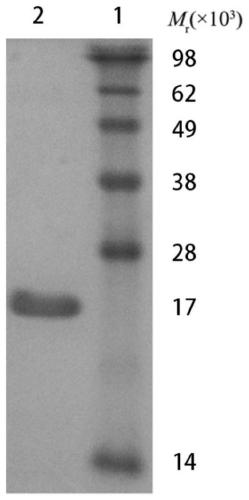
[0038] Example 1 Expression and identification of the C-terminal peptide of Borrelia garzii OspA protein
[0039] 1. Cloning and expression of OspA gene fragment
[0040] The gene fragment of the outer membrane protein OspA 126-274aa of Chinese Lyme spirochete B.garinii genotype PD91 (that is, the cross-reactive sequence has been removed from the full-length sequence of the OspA protein), with EcoRI and HindIII restriction sites added at both ends, and Cloned and expressed after being fused with the His tag, the nucleic acid sequence of the OspA gene fragment is shown in SEQ ID NO:2.
[0041] The primers used are as follows:
[0042] Upstream primer: 5'- GAATTC TTTAATGCAAAAGGTGA-3' (underlined as EcoRI restriction site);
[0043] Downstream primer: 5'- AAGCTT TTATTTTAAAGCTGTTTTAAG-3' (underlined HindIII restriction site).
[0044]The OspA gene fragment and the plasmid PET-30a were subjected to a double digestion reaction, and the two double digestion products were ligat...
Embodiment 2
[0049] Example 2 Immunoprotective study of rOspA-pep protein
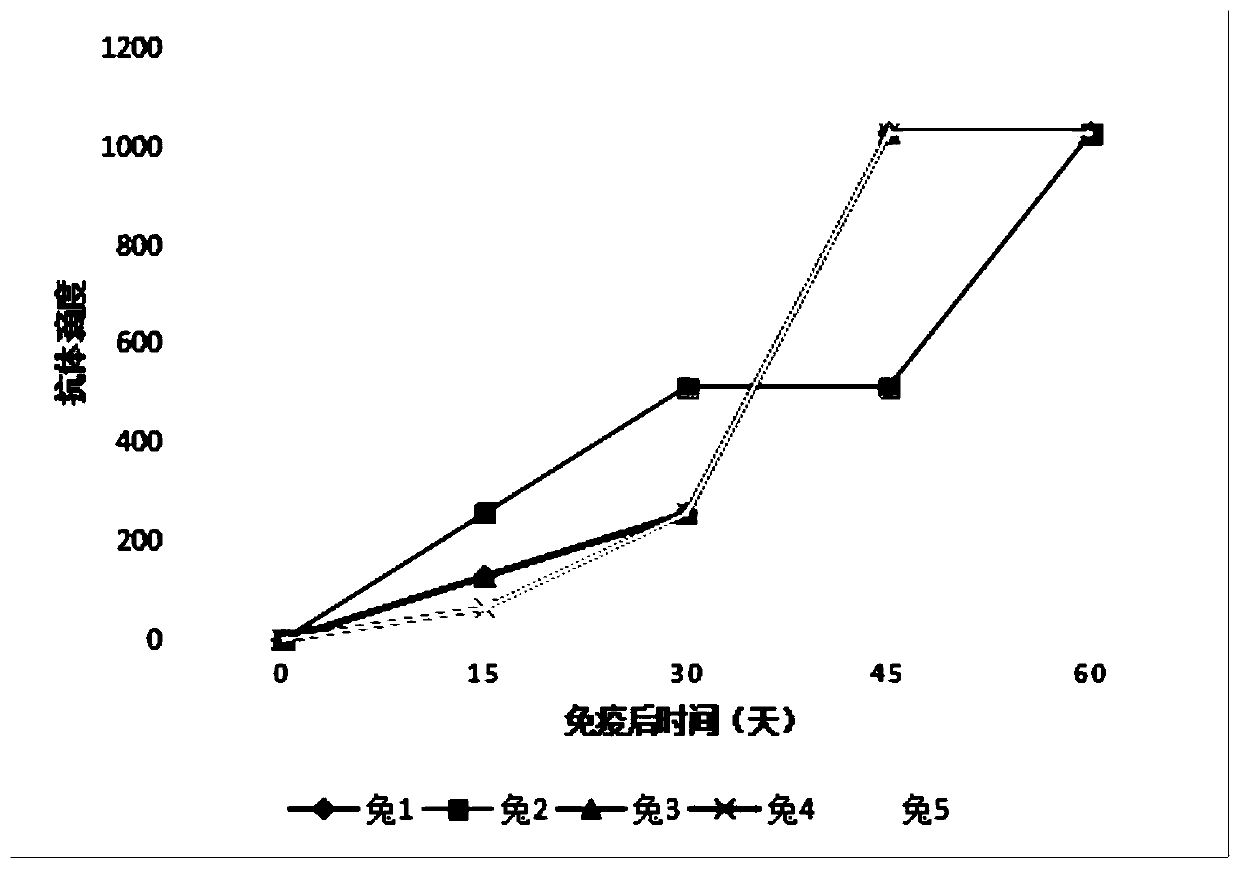
[0050] 1. Screening of optimal immune dose of rOspA-pep protein
[0051] Set up 7 dose groups (20, 30, 40, 50, 60, 80, 100 μg) and 1 blank control group. Dosage groups were immunized with New Zealand rabbits (5 rabbits in each group) with different doses of rOspA-pep protein, and the control group was injected with the same volume of PBS to screen the optimal immune dose of rOspA-pep. The IgG antibody titer of rabbit serum was detected before immunization and on the 15th, 30th, 45th and 60th day after immunization by indirect immunofluorescence (IFA) method. The rabbit serum of the dose group with higher titer was selected for further neutralization test. Select 10 New Zealand rabbits (body weight 2.5kg, half male and half male) to be immunized with rOspA-pep protein at the optimal dose, and the other 5 rabbits were used as blank control group (the control group was injected with PBS solution). The first immuniz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com