B.garinii polyclonal antibody and application
A technology of Borrelia garzii and polyclonal antibody, which is applied in the direction of anti-bacterial immunoglobulin, resistance to vector-borne diseases, immunoassay, etc., can solve the problems of easy cross-contamination, long culture period, and horizontal comparison of the effectiveness of PCR methods and other issues, to achieve the effect of good specificity, strong application prospects, and high potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1. Preparation of Borrelia garzii polyclonal antibody
[0024] (1) Antigen preparation: Borrelia garinii B.garinii NMJW1 strain was inoculated into 50ml of BSK-II medium (Sigma-Aldrich, USA), and cultivated in a 33°C, 5% CO2 constant temperature incubator until the cell concentration was 2× 108cfu / ml, to obtain the culture solution; then the culture solution was centrifuged at 4°C, 3200rcf for 25min, the precipitate was collected and washed twice with 0.9% NaCl aqueous solution with a mass concentration of 0.9%; After suspending the bacteria, incubate in a water bath at 60°C for 1 hour, and shake it from time to time; centrifuge at 3200 rcf for 25 minutes at 4°C, collect the bacterial precipitate, wash with 0.9% NaCl aqueous solution three times, and obtain the antigen of Borrelia garzii;
[0025] (2) Animal immunization: resuspend the obtained Borrelia garzii with 10ml of 0.9% NaCl aqueous solution, and the concentration of the whole bacterial antigen suspensio...
Embodiment 2
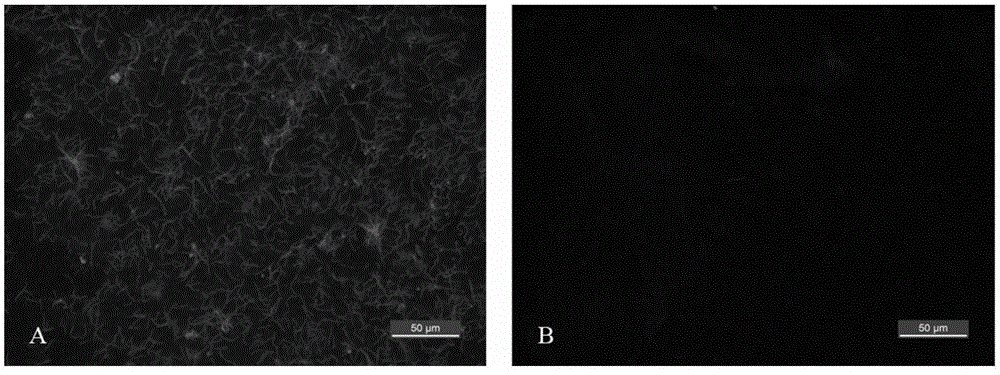
[0028] Example 2. Immunofluorescence detection of Borrelia Lyme disease
[0029] (1) Borrelia garinii B.garinii NMJW1 bacterial strain is inoculated in 15ml BSK-II culture medium (Sigma-Aldrich Company of the U.S.A.), cultivated to bacterium concentration 5×107cfu / ml at 33 ℃, 5% CO2 constant temperature incubator , to obtain cell culture fluid;
[0030] (2) Centrifuge the bacterial cell culture solution in step (1) at 4° C. at 3200 rcf for 25 min, collect the precipitate and resuspend and wash it with 15 ml of 0.9% NaCl aqueous solution with a mass concentration of 15 ml, repeat twice, and finally obtain the bacterial cells by centrifugation;
[0031] (3) Resuspend the bacterial cells obtained in step (2) with 10 ml of 0.9% NaCl aqueous solution, take 10 μl of the suspension and spread it evenly on the immunohistochemical glass slide, and let it dry naturally for 10 min;
[0032] (4) Fix the immunohistochemical slides obtained in step (3) at 60° C. for 30 min;
[0033] (5) F...
Embodiment 3
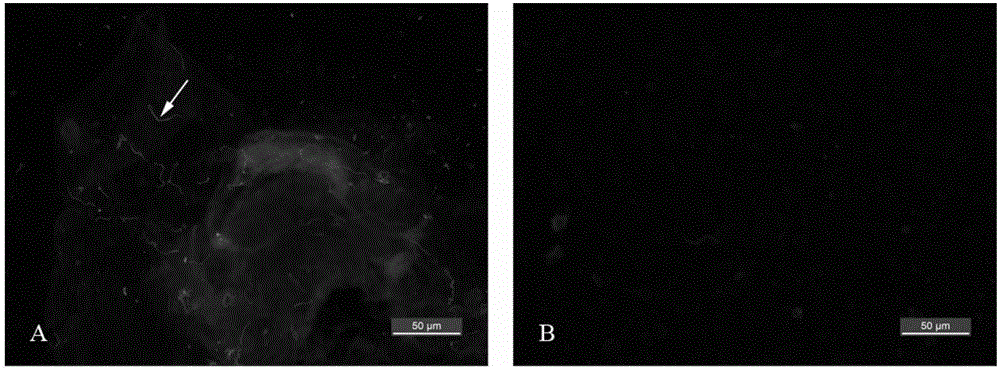
[0040] Example 3. Immunofluorescence detection of Borrelia Lyme disease in the midgut of larval ticks
[0041] (1) Collect the larvae that fell after being full of blood, and randomly take 10 midgut tissues for dissection;
[0042] (2) After the midgut tissue was washed 3 times with PBS solution, it was placed on an immunohistochemical slide and dried naturally for 20 min;
[0043] (3) Fix the immunohistochemical slide obtained in step (2) at 60° C. for 30 min;
[0044] (4) Immunohistochemical slides were fixed in acetone for 5 minutes, and dried at room temperature for 20 minutes;
[0045] (5) Add 100 μl of PST blocking solution to the midgut tissue, place the immunohistochemical slide in a sealed black box and keep it moist, and incubate at 37° C. for 30 minutes; the PST blocking solution contains 5 g of bovine serum albumin per 100 ml, 50μl Tween-20 in PBS solution;
[0046] (6) Remove the PST blocking solution, add 100 μl of primary antibody working solution, and incuba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com