Tandospirone hydrochloride crystal form I and preparation method thereof
A technology of tandospirone hydrochloride and tandospirone, which is applied in the field of tandospirone hydrochloride crystal form I and its preparation, can solve problems such as the report of tandospirone hydrochloride crystal form, and achieve water solubility and stability Good, high yield, enhanced bioavailability and safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 Preparation method of tandospirone hydrochloride crystal form I
[0041]Weigh 500g of tandospirone, add 12.5L of a mixed solution of acetone and ether (40:60 in volume ratio), heat up to 40°C, and after the dissolution is complete, slowly add 0.13L of 10mol / L aqueous hydrochloric acid solution dropwise, drop Stop heating after finishing, let cool to room temperature naturally for 12 hours, filter with suction, wash, and dry to obtain 519 g of tandospirone hydrochloride crystal form I with a yield of 95.0%.
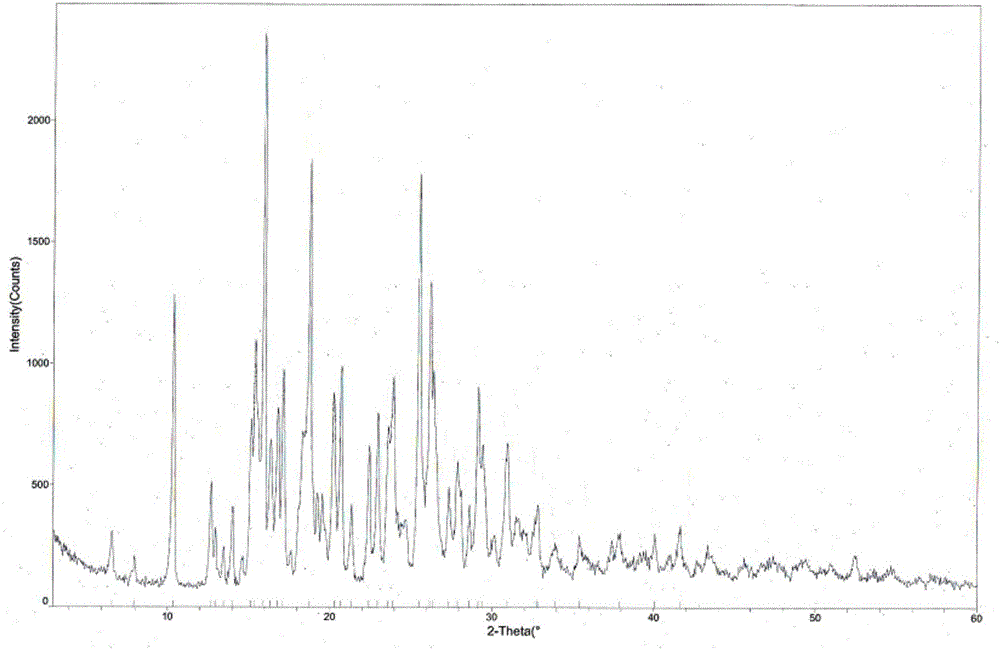
[0042] The measured melting point of tandospirone hydrochloride crystal form I is 224.0-225.0°C, and the X-ray powder diffraction pattern is shown in figure 1 (Using DX-2700 X-ray powder diffractometer to analyze the crystal phase of the sample, Cu Kα radiation, tube voltage 40KV, tube current 30mA), the relevant diffraction data are shown in Table 1 (2θ measurement error is ±0.2), and the infrared spectrum is shown in Figure 4 .
[0043] Table 1 X-r...
Embodiment 2
[0045] Embodiment 2 The preparation method of tandospirone hydrochloride crystal form I
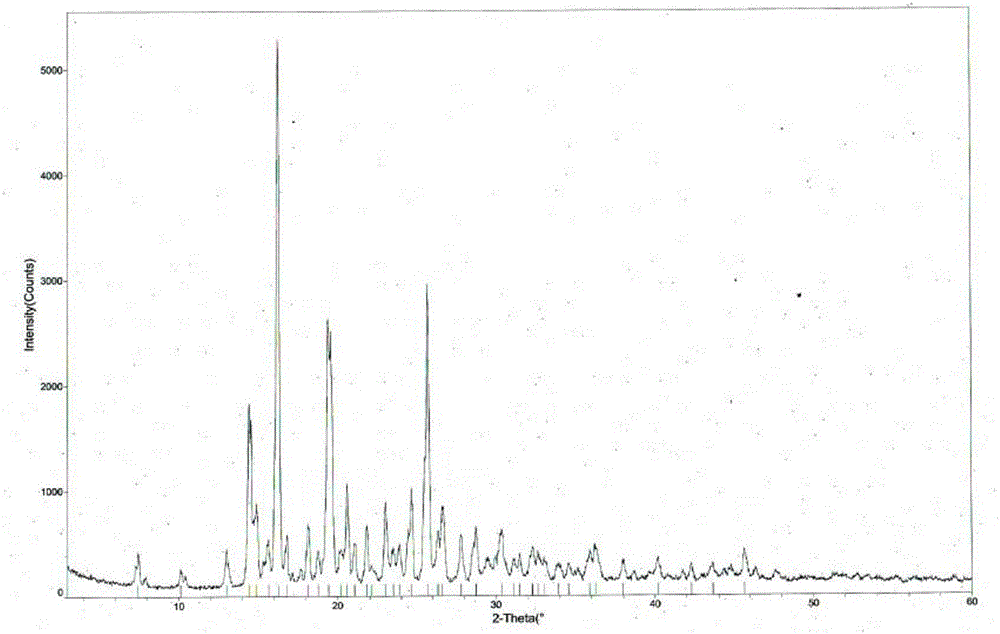
[0046] Weigh 500g of tandospirone, add 8.0L of a mixed solution of acetone and ether (65:35 in volume ratio), heat up to 50°C, after the dissolution is complete, add 0.2L of 10mol / L aqueous hydrochloric acid solution dropwise, Afterwards, stop heating, cool naturally to room temperature for 2 hours, then place at -5±5°C for 8 hours, filter with suction, wash, and dry to obtain 513 g of tandospirone hydrochloride Form I, with a yield of 94.0%. The structural analysis results of the obtained product are not significantly different from those of Example 1.
Embodiment 3
[0047] Embodiment 3 Preparation method of tandospirone hydrochloride crystal form I
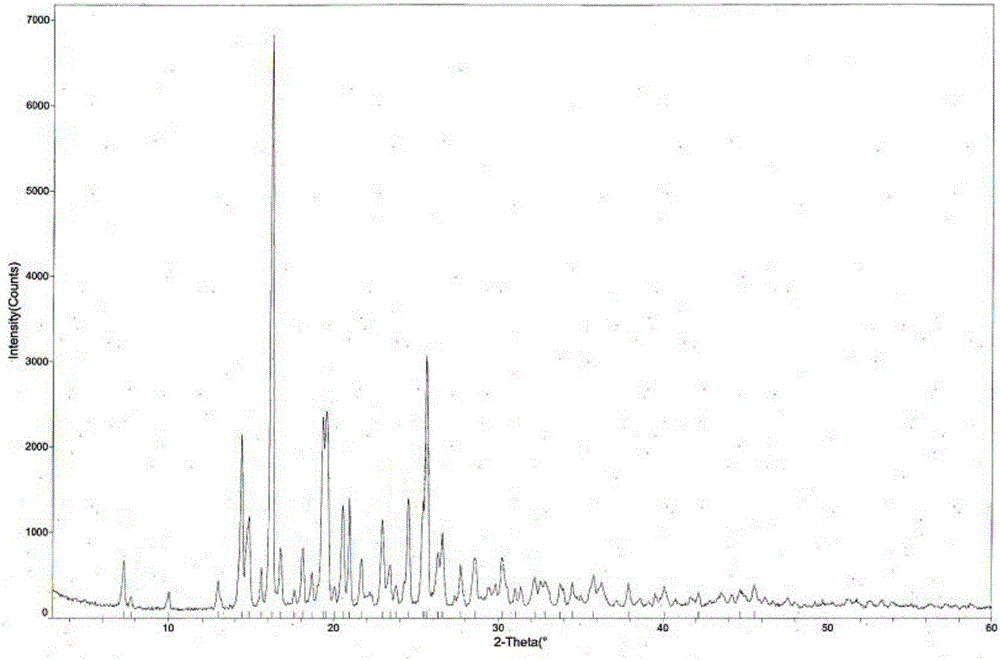
[0048] Weigh 500g of tandospirone, add 5.0L of a mixed solution of acetone and ether (80:20 by volume), heat up to 30°C, and after the dissolution is complete, add 0.26L of 10mol / L aqueous hydrochloric acid solution dropwise, Afterwards, stop heating, cool naturally to room temperature for 6 hours, then place at -5±5°C for 5 hours, filter with suction, wash, and dry to obtain 516 g of tandospirone hydrochloride crystal form I with a yield of 94.5%. The structural analysis results of the obtained product are not significantly different from those of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com