Preparation method of nevirapine intermediate spherical crystal
A spherical crystal, nevirapine technology, applied in the field of medicinal chemistry, to achieve the effects of good reproducibility of the preparation process, high crystallization yield and high crystal purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The preparation method of the compound II reaction liquid in the present invention refers to the synthesis methods disclosed in patents US5569760, DE4403311, WO2007010352, and US5366972, and the method is as follows:
[0046] 117.5g of 2-chloro-N-(2-chloro-4-methyl-3-pyridyl)-3-pyridinecarboxamide, 47.5g of cyclopropylamine, 46.7g of calcium oxide, 125-375ml of organic solvent (the organic solvent can be Any one selected from toluene, xylene, dioxane, ethylene glycol dimethyl ether or N,N-dimethylformamide) is added into a pressure reactor, and the temperature is controlled at 135-140° C. to stir and react. After the reaction was completed, the temperature was lowered to 100-120°C, and the calcium oxide filter residue was removed by filtration to obtain 125 g of compound II (11-cyclopropyl-5,11-dihydro-4-methyl-6 hydrogen-bipyridine-[3,2 -b: a reaction solution of 2,3-e][1,4]diazepine).
Embodiment 1
[0048] The xylene (125ml) reaction solution containing 125g of compound II was heated to reflux to dissolve. After dissolving and clearing, the reaction solution was slowly added dropwise to 125ml of xylene at 5-10°C, and the temperature of the crystallization solution was controlled at 20-40°C during the dropping process. After the dropwise addition, the temperature was lowered to -5~5°C. After filtration, vacuum drying at 90°C, and discharge, 114.0 g of spherical Compound II crystals were obtained, with an HPLC purity of 99.1% and a yield of 91.2%. result:
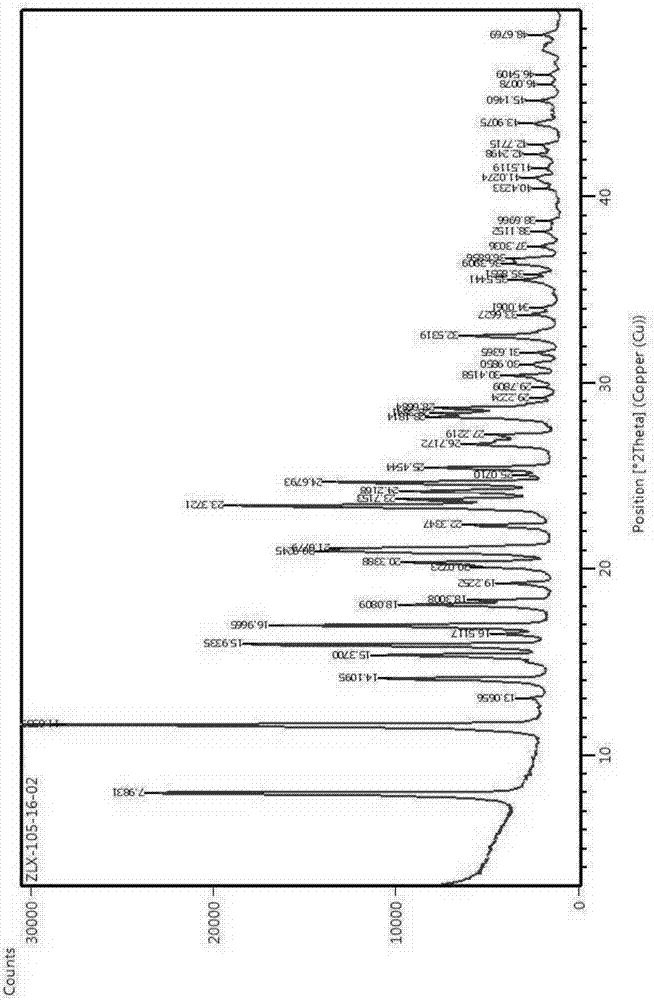
[0049] HPLC test, DSC test and XRD test were performed on the obtained compound II crystal.
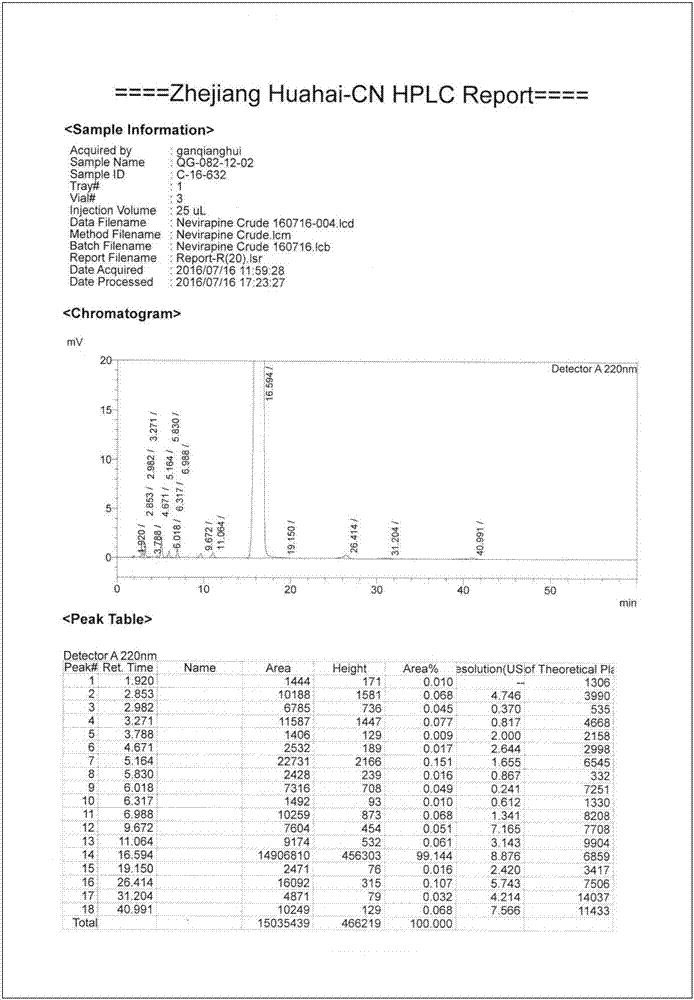
[0050] figure 1 It is the compound II crystal HPLC collection of patterns that embodiment 1 obtains, from figure 1 As can be seen in the figure, the purity of the crystal reaches 99.1%.
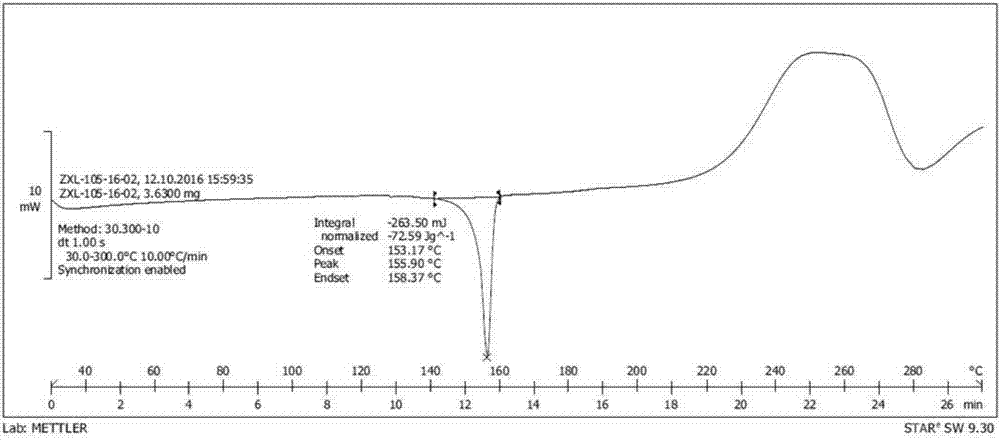
[0051] figure 2 It is the DSC collection of illustrative plates of the compound II crystal that embodiment 1 obtains, from figure 2...
Embodiment 2
[0056] The xylene (250ml) reaction solution containing 125g of compound II was heated to reflux to dissolve. After dissolving and clearing, the reaction solution was slowly added dropwise into 62.5ml of xylene at 5-10°C, and the temperature of the crystallization solution was controlled at 20-30°C during the dropping process. After the dropwise addition, the temperature was lowered to -5~5°C. After filtration, vacuum drying at 90°C, and discharge, 107.0 g of spherical Compound II crystals were obtained, with an HPLC purity of 99.3% and a yield of 85.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com