Synthesis method of suvorexant intermediate
A synthetic method and intermediate technology, applied in the field of drug synthesis, can solve problems such as complex process routes, poor atom economy, and harsh reaction conditions, and achieve the effects of high reaction yield, high production safety, and fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
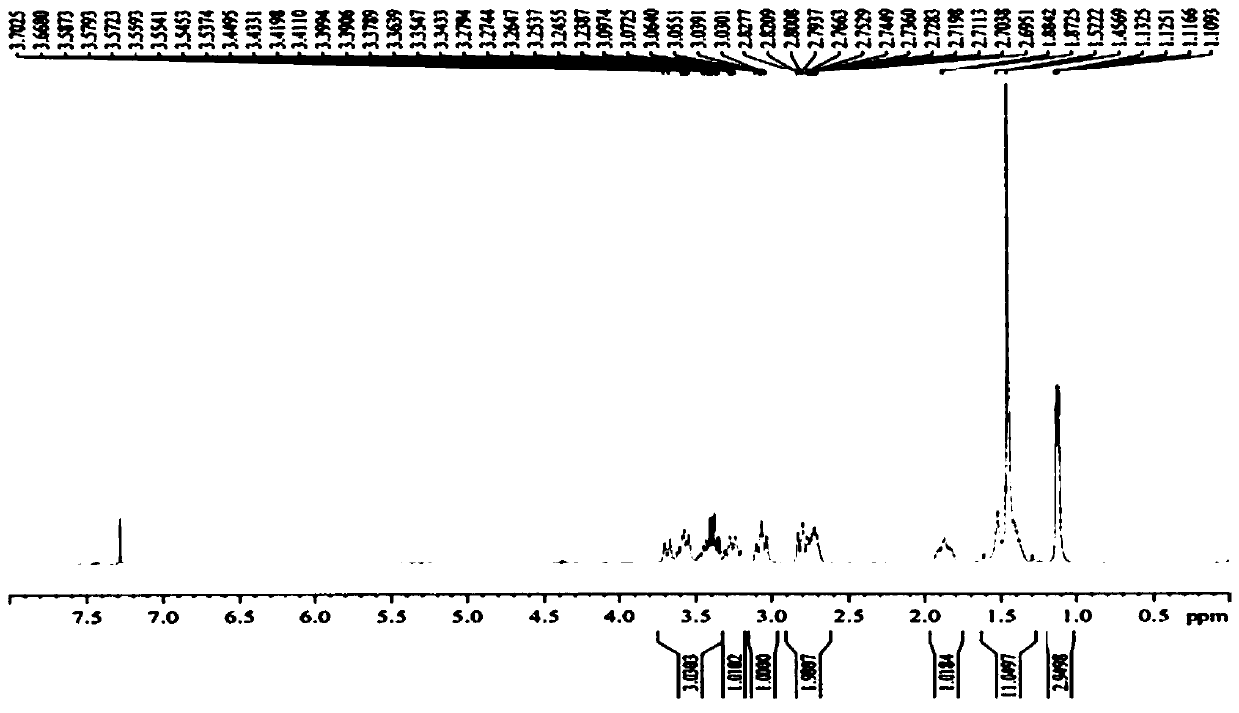
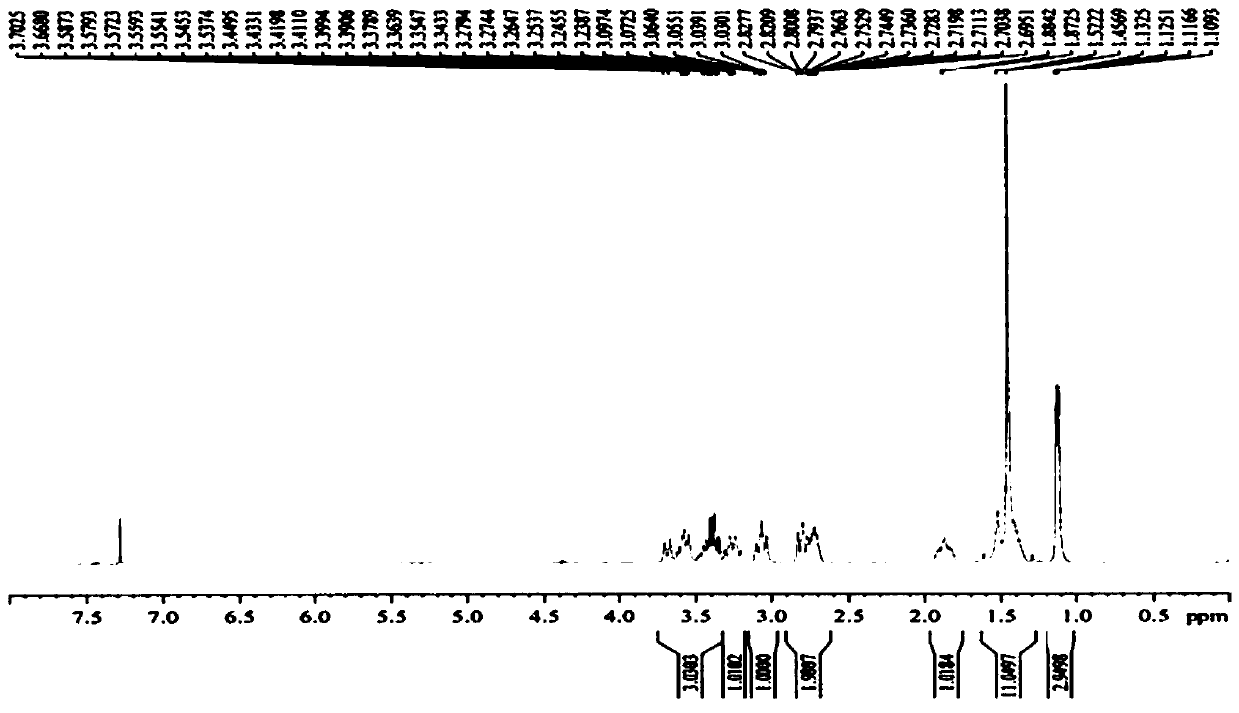
[0047] Example 1 Synthesis of Suvorexant intermediate (5S)-hexahydro-5-methyl-1H-1,4-diazepine-1-carboxylic acid tert-butyl ester
[0048] Step 1: Compound Suvor-2 Synthesis
[0049] Feeding table 1-1
[0050]
[0051] 400 g of solvent tetrahydrofuran and 50 g of raw material compound Suvor-1 were sequentially added into the reaction kettle, and the temperature was lowered to -10 to 0°C. Add 75g of red aluminum solution in batches, control the temperature at -10°C to 0°C, react until the raw materials are monitored by TLC after the addition is complete, add 200g of 10% potassium sodium tartrate solution and 200g of ethyl acetate, stir until the system is clear, and separate the liquids. Collect the organic phase. Concentrated under reduced pressure to obtain compound Suvor-2, weighing 41.8 g, HPLC purity 98%, yield 88%.
[0052] Step 2: Synthesis of Compound Suvor-3
[0053] Feeding table 1-2
[0054]
[0055] Dissolve 26.4g of compound Suvor-2 in 132g of tetrahydro...
Embodiment 2
[0068] Example 2 Synthesis of Suvorexant intermediate (5S)-hexahydro-5-methyl-1H-1,4-diazepine-1-carboxylic acid tert-butyl ester 1: Synthesis of compound Suvor-2
[0069] Feeding table 2-1
[0070]
[0071]
[0072] 300 g of solvent tetrahydrofuran and 50 g of raw material compound Suvor-1 were sequentially added into the reaction kettle, and the temperature was lowered to -10 to 0°C. Add 50g of red aluminum solution in batches, and control the temperature at -10°C to 0°C. After the addition is complete, react until the raw materials monitored by TLC disappear, add 100g of 10% potassium sodium tartrate solution and 100g of ethyl acetate, stir until the system is clear, and separate the liquids. Collect the organic phase. Concentrated under reduced pressure to obtain compound Suvor-2, weighing 40.4 g, HPLC purity 97%, yield 85%.
[0073] Step 2: Synthesis of Compound Suvor-3
[0074] Feeding table 2-2
[0075]
[0076] Dissolve 26.4g of compound Suvor-2 in 79g of ...
Embodiment 3
[0089] Synthesis of Example 3 (5S)-hexahydro-5-methyl-1H-1,4-diazepine-1-carboxylic acid tert-butyl ester
[0090] Step 1: compound Suvor-2 synthesis
[0091] Feeding table 3-1
[0092]
[0093]500 g of solvent tetrahydrofuran and 50 g of raw material compound Suvor-1 were successively added into the reaction kettle, and the temperature was lowered to -10 to 0°C. Add 100g of red aluminum solution in batches, and control the temperature at -10°C to 0°C. After the addition is complete, react until the raw materials are monitored by TLC. Add 300g of 10% potassium sodium tartrate solution and 300g of ethyl acetate, stir until the system is clear, and separate the liquids. Collect the organic phase. Concentrate under reduced pressure to obtain the intermediate Suvor-2, weighing 40.6 g, with an HPLC purity of 98% and a yield of 85%.
[0094] Step 2: Synthesis of Compound Suvor-3
[0095] Feeding table 3-2
[0096]
[0097] Dissolve 26.4g of compound Suvor-2 in 264g of tet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com