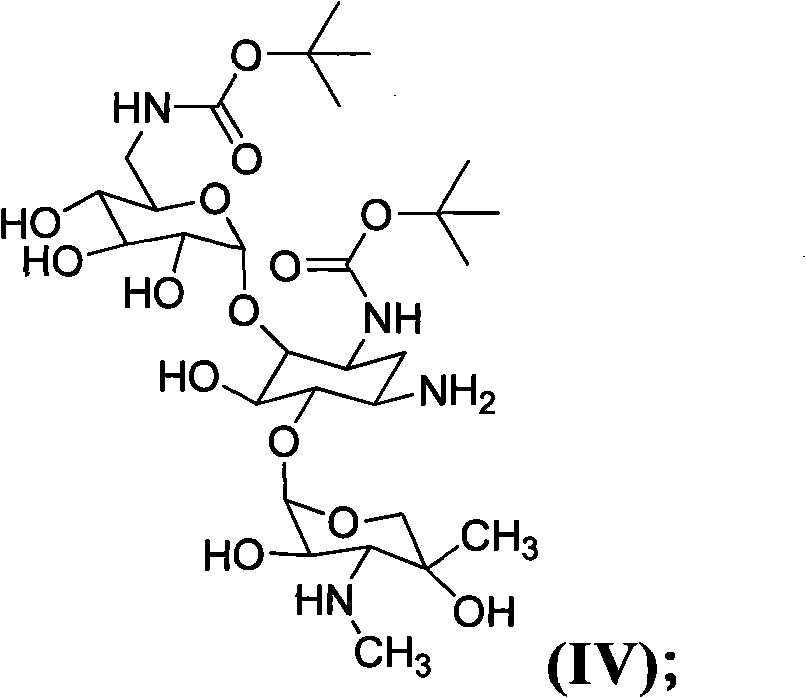
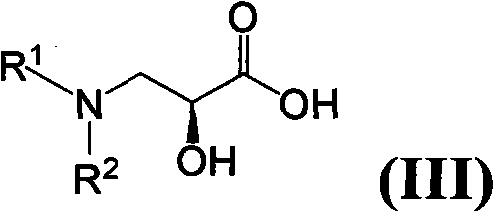
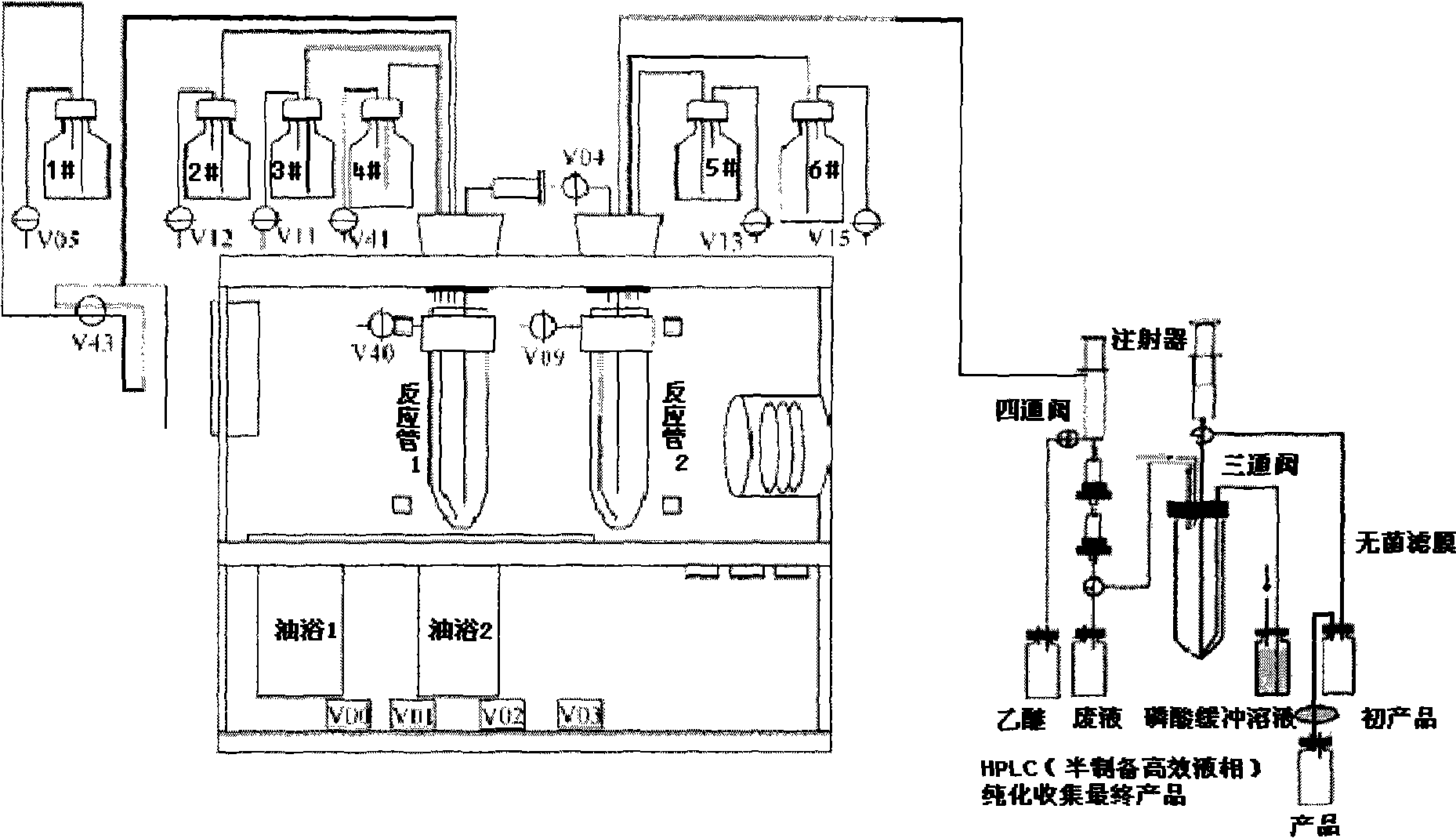
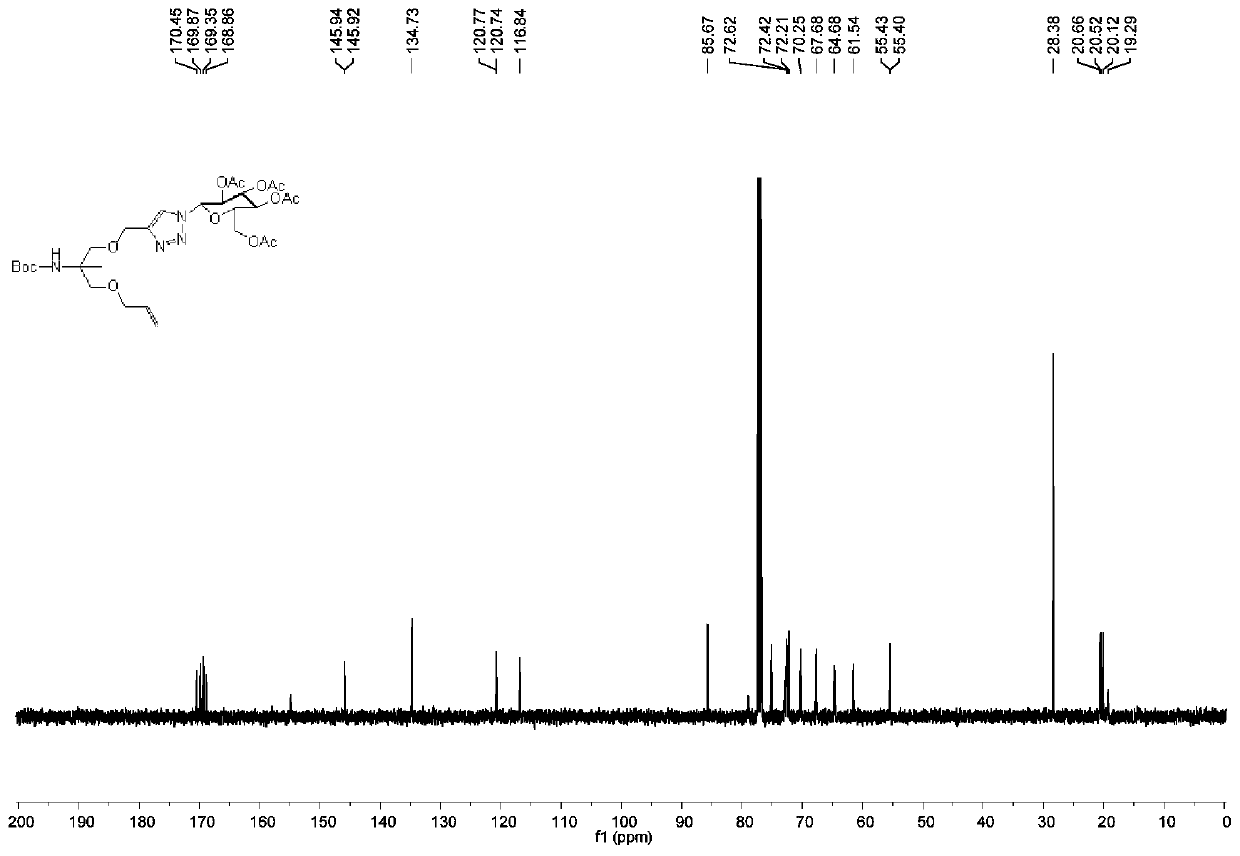
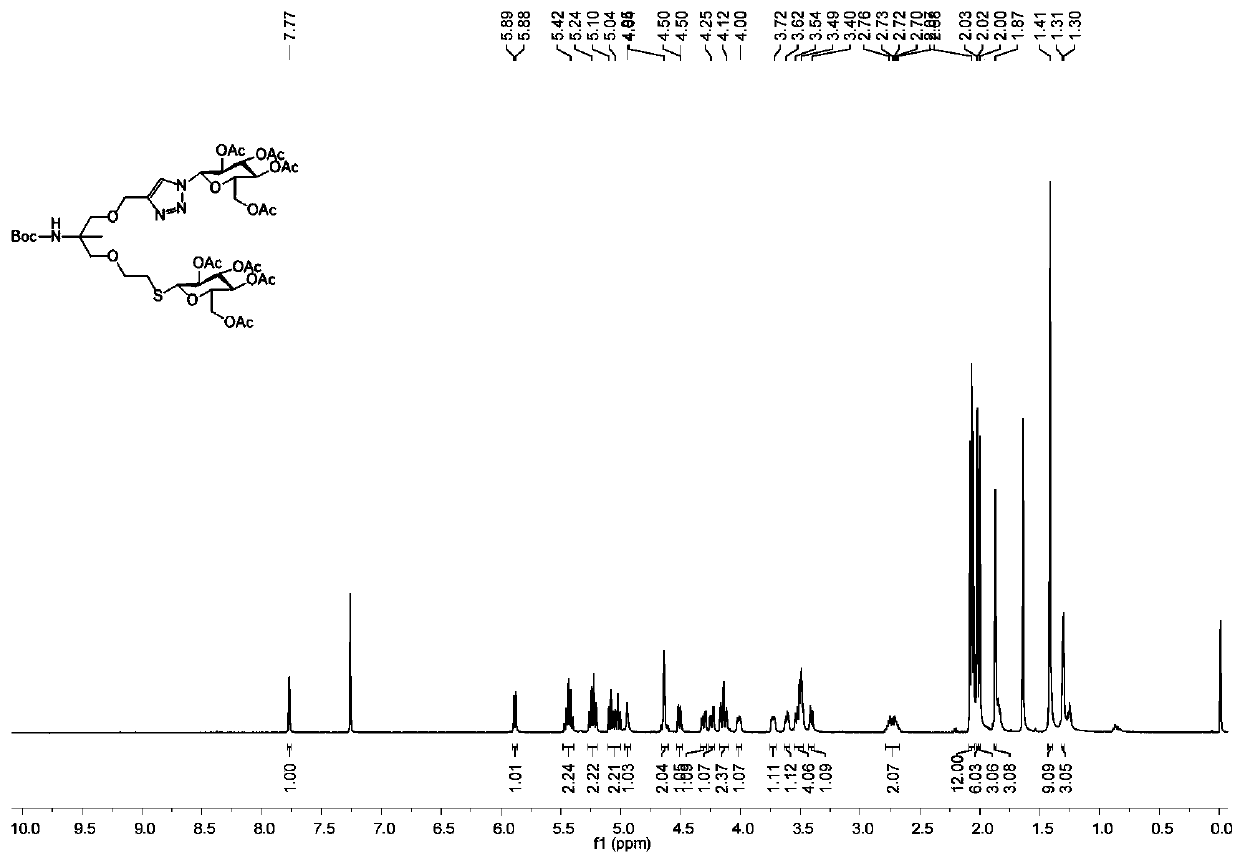
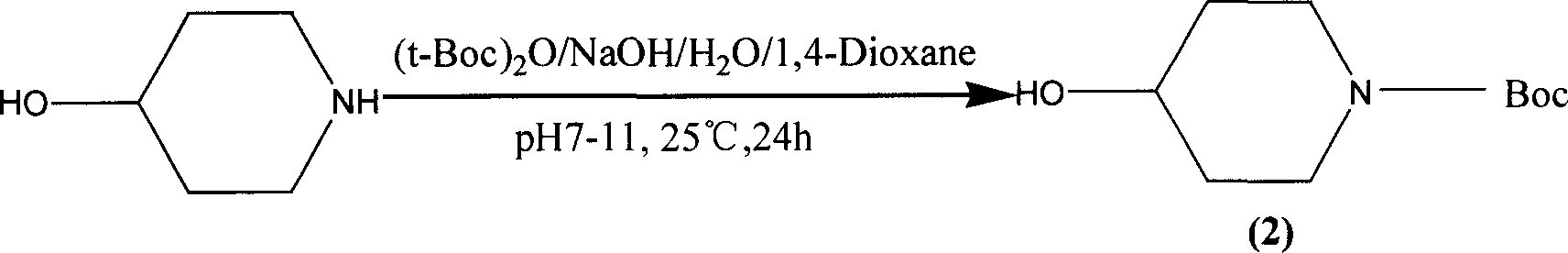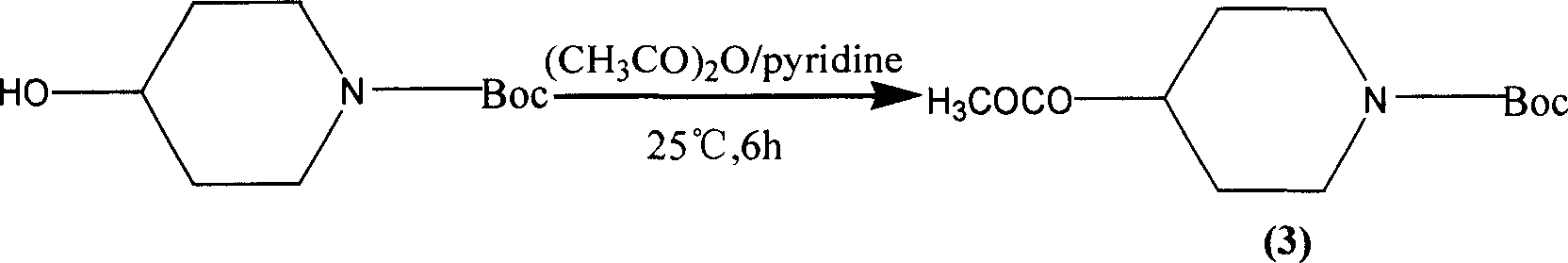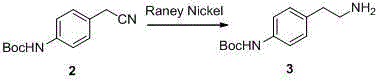Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
296 results about "Di-tert-butyl dicarbonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. Since this compound can be regarded formally as the acid anhydride derived from a tert-butoxycarbonyl (Boc) group, it is commonly referred to as "Boc anhydride." This pyrocarbonate reacts with amines to give N-tert-butoxycarbonyl or so-called Boc derivatives. These carbamate derivatives do not behave as amines, which allows certain subsequent transformations to occur that would be incompatible with the amine functional group. The Boc group can later be removed from the amine using moderately strong acids (e.g., trifluoroacetic acid). Thus, Boc serves as a protective group, for instance in solid phase peptide synthesis. Boc-protected amines are unreactive to most bases and nucleophiles, allowing for the use of the fluorenylmethyloxycarbonyl group (Fmoc) as an orthogonal protecting group.
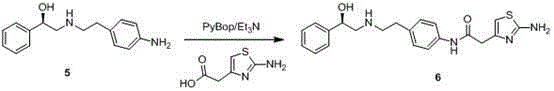
Tulathromycin intermediate and preparation method thereof, as well as preparation method of tulathromycin
ActiveCN102786569AReduce manufacturing costMild conditionsSugar derivativesSugar derivatives preparationEpoxyTert-Butyloxycarbonyl protecting group
The invention provides a tulathromycin intermediate, a preparation method of the tulathromycin intermediate, and a preparation method of the tulathromycin. The preparation method of the tulathromycin has the advantages of mild condition, convenience for operation, and low cost. The preparation method of the tulathromycin comprises the following steps of: using azithromycin A as a raw material; protecting 2'-hydroxy and 6'-amino in the azithromycin A through di-tert-butyl dicarbonate so as to obtain double-protective azithromycin A; carrying out Swern oxidation to 4''-hydroxy to the double-protective azithromycin A; salifying along with trifluoroacetic acid; and synchronously removing boc t-butyloxycarbonyl to obtain the azithromycin A bitrifluoroacetic acid salt of 4''-carbonyl; and then reacting with trimethylsulfonium bromide to obtain 4''-epoxy compound; and finally carrying out nucleophilic addition on the 4''-epoxy compound by n-propylamine so as to obtain the phosphate of tulathromycin; and further neutralizing via alkaline to obtain the target compound tulathromycin; and synchronously obtaining the tulathromycin intermediate of azithromycin A bitrifluoroacetic acid salt of 4''-carbonyl.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
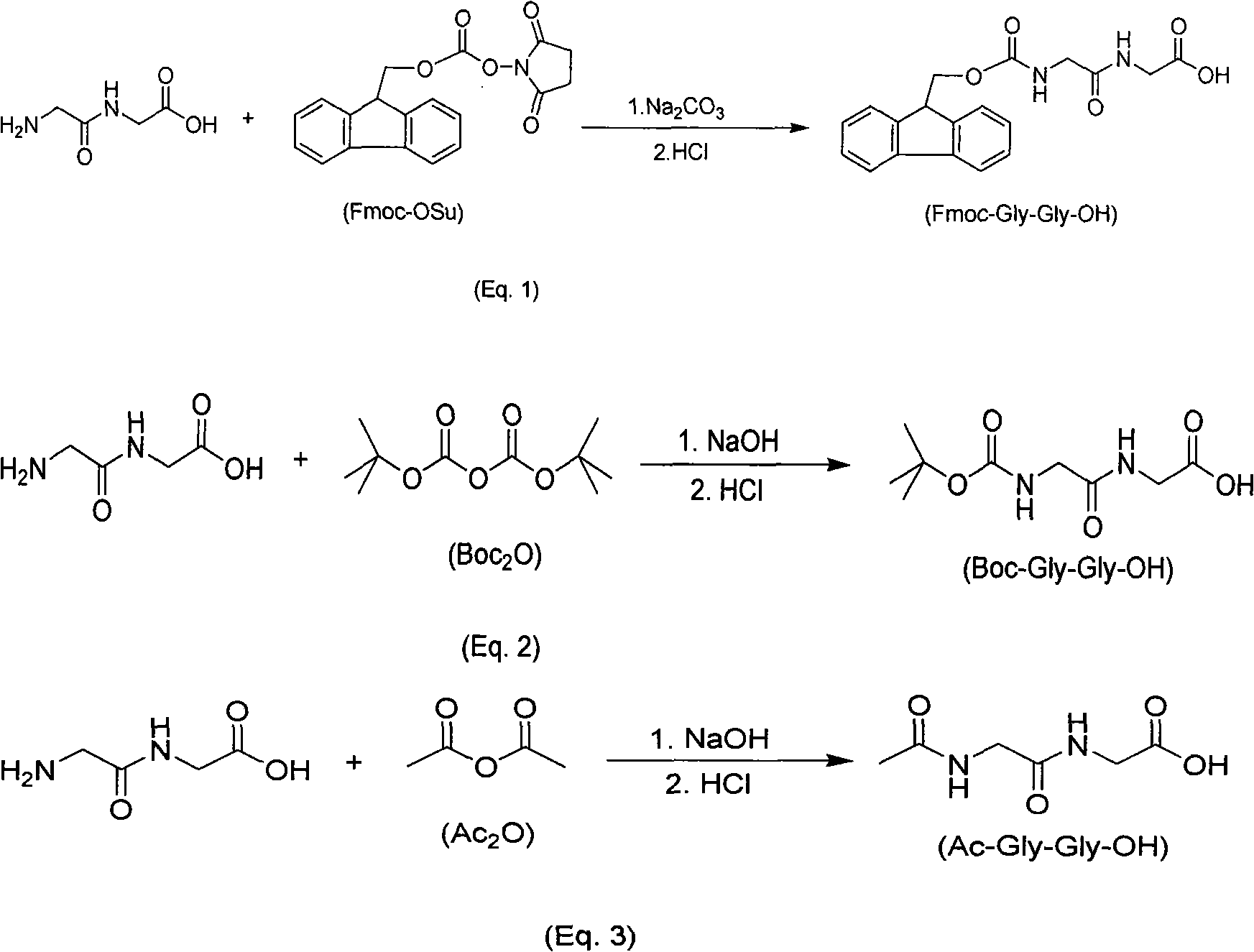
Synthesis method of amino-protecting glycine dipeptidase derivant
The invention relates to a synthesis method of amino-protecting glycine dipeptidase derivant. The amino-protecting glycine dipeptidase derivant is N-9-Fmoc glycine dipeptidase, N-Boc glycine dipeptidase and N-acetyl glycine dipeptidase. The invention has the technology that glycine dipeptidase is dissolved in 1-20% of inorganic aqueous alkali, and the organic solution of Fmoc-OSu or Boc2O or Ac2Ois dropwise added; reaction is finished within 1-12 hours, the yield of the product Fmoc-Gly-Gly-OH, Boc-Gly-Gly-OH and Ac-Gly-Gly-OH is between 80-95%, and the content of the product is above 99%. The invention has simple technology, easy industrialization and low cost. The product of the invention is the important raw material for synthesizing polypeptide compound.
Owner:上海力智生化科技有限公司
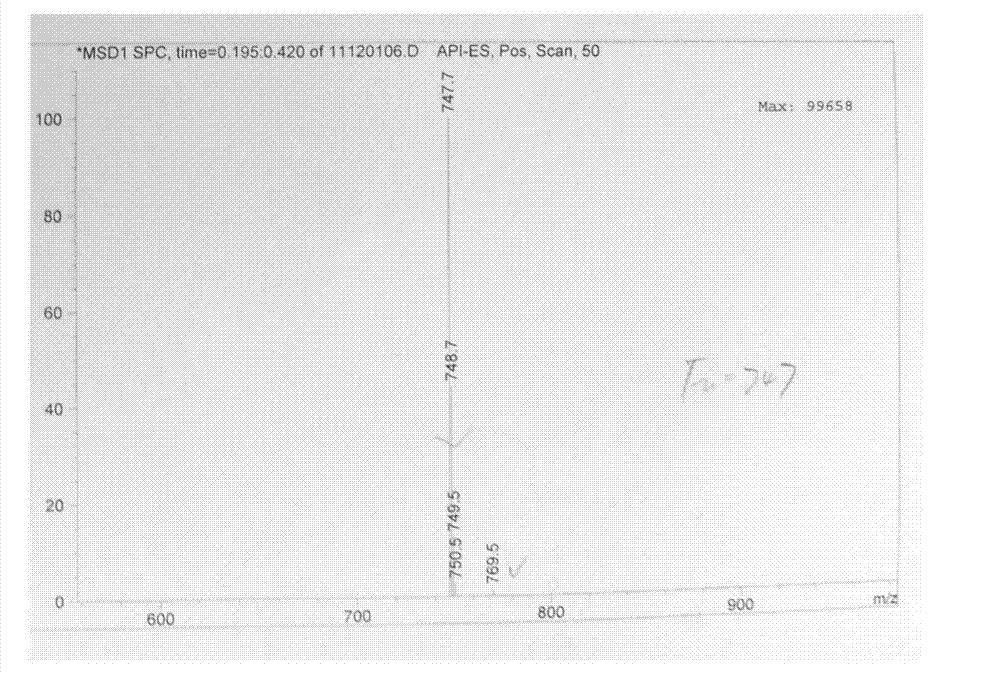
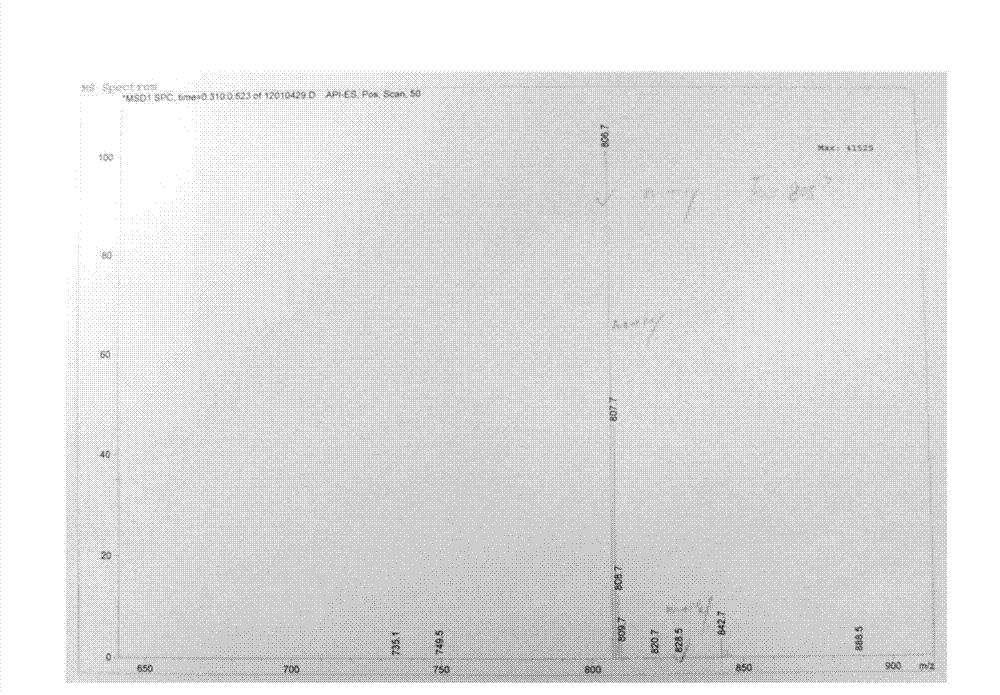
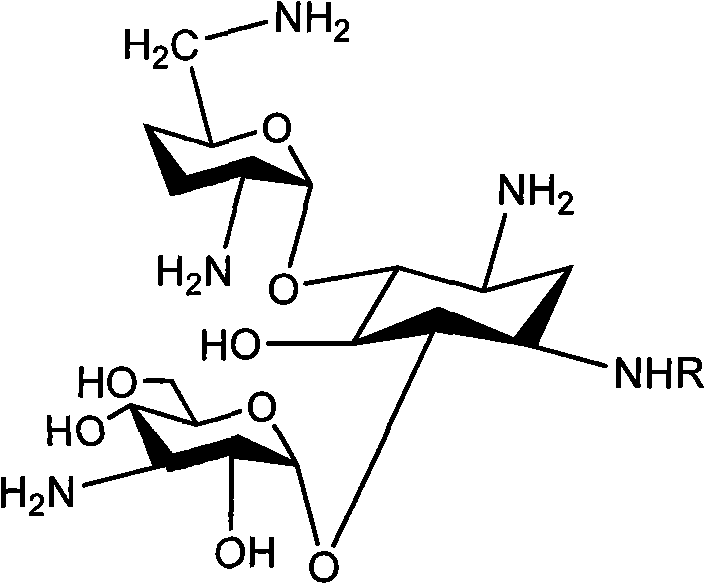
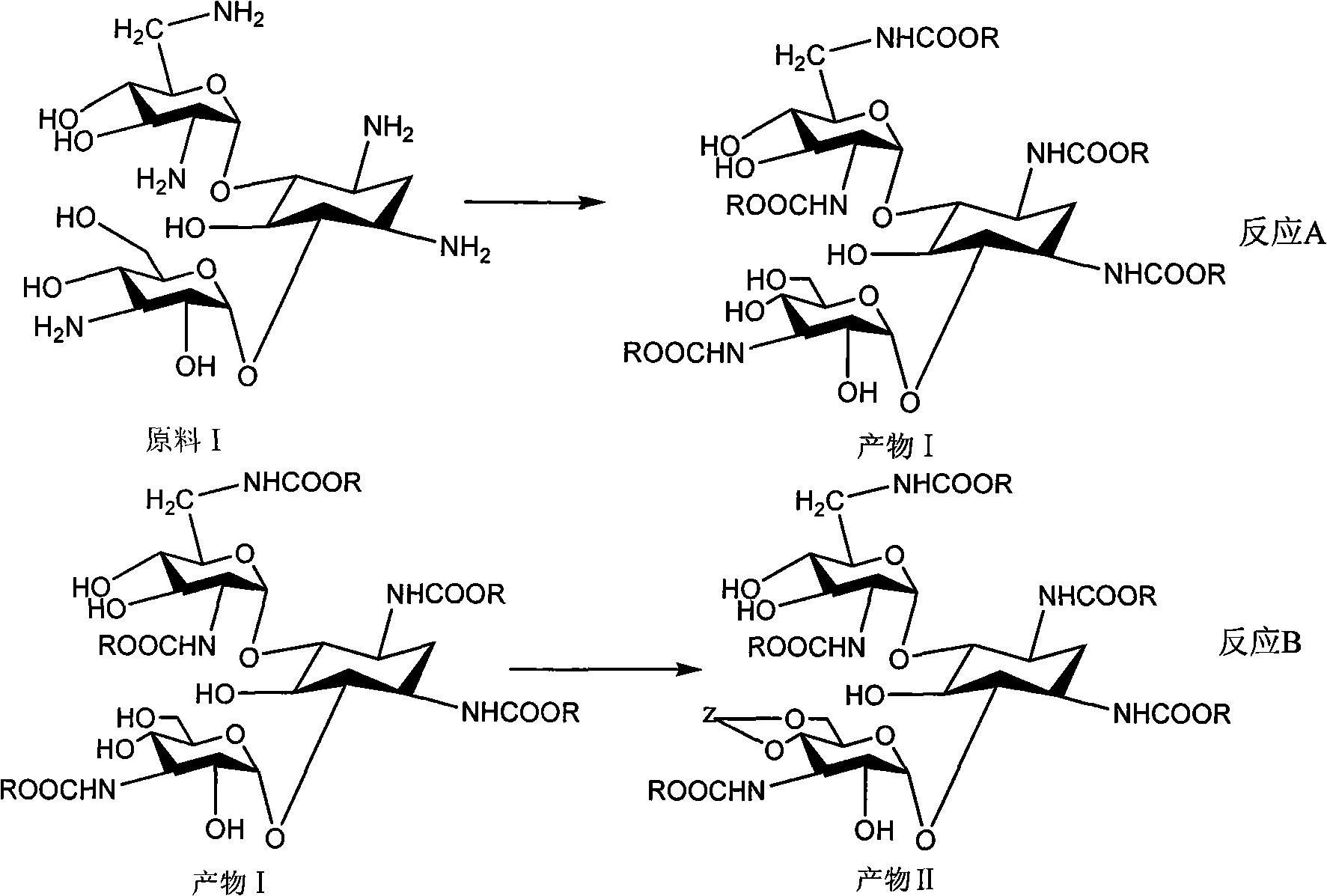
Method for synthesizing Arbekacin and intermediate dibekacin thereof
InactiveCN101575354AHigh yieldReduce generationSugar derivativesSugar derivatives preparationFluoroacetic acidSynthesis methods
The invention relates to an organic synthesis method, in particular to a method for synthesizing an Arbekacin and an intermediate dibekacin thereof. The method comprises the following steps of: takinga kanamycin B as initial raw material, carrying out the following processes of aldol condensation, sulfonylation, sodium iodide replacement and elimination to form double bond, de-protection under acidic condition, amino-electron reduction and final hydrogenation, thus obtaining the dibekacin; taking 3',4'-dideoxy -3',4'-didehydro-kanamycin B as raw material, using a di-tert-butyl dicarbonate toselectively protect the amidogen of 3, 2', 6', 3'' sites; subsequently using the synthesized active ester to protect the 1-site amidogen; subsequently using tri-fluoroacetic acid to remove BOC; and carrying out hydrazinolysis and catalysis and hydrogenation of platinum oxide, thus obtaining the Arbekacin. The synthesis method has the advantages of simple operation, high outcome yield, reducing thecost of raw material, optimizing the reaction route, lowering the requirements to the reaction conditions and being beneficial to industrial production.
Owner:BEIJING UNIV OF CHEM TECH +1
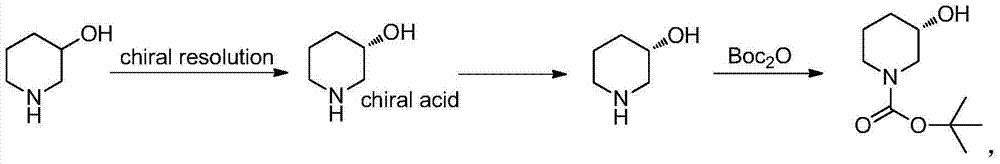
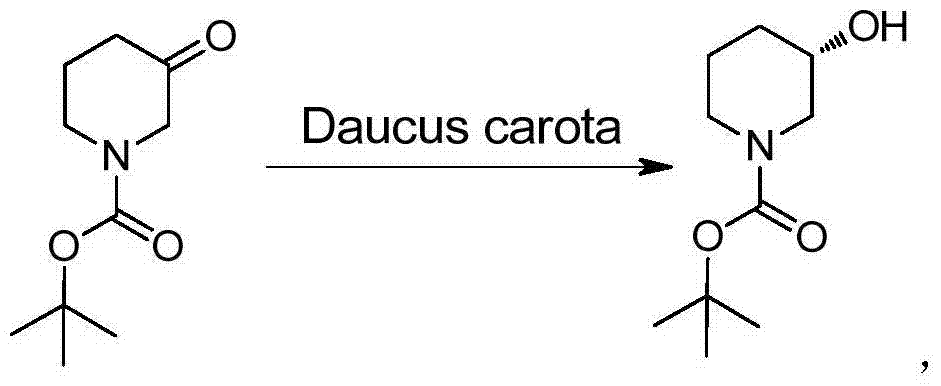
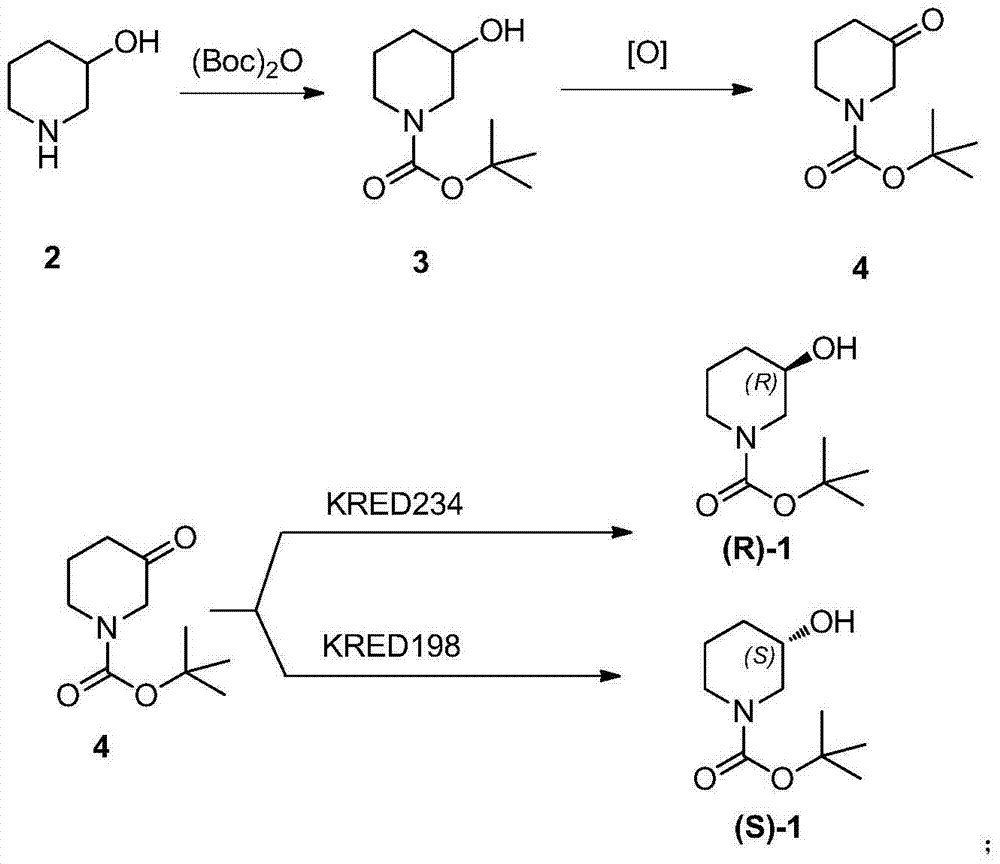
Method for preparing chiral N-tert-butyloxycarboryl-3-hydroxypiperidine
InactiveCN103571908ARaw materials are cheap and easy to getMild reaction conditionsFermentationTert-Butyloxycarbonyl protecting groupPyridine
The invention discloses a method for preparing chiral N-tert-butyloxycarboryl-3-hydroxypiperidine. The method comprises the following steps: a) enabling racemic 3-hydroxypiperidine and di-tert-butyl dicarbonate ester to react to generate racemic N-tert-butyloxycarboryl-3-hydroxypiperidine; b) oxidizing the racemic N-tert-butyloxycarboryl-3-hydroxypiperidine into racemic N-tert-butyloxycarboryl-3-piperidone under the action of an oxidizing agent; c) reducing the racemic N-tert-butyloxycarboryl-3-piperidone by using a ketoreductase into the chiral N-tert-butyloxycarboryl-3-hydroxypiperidine. Compared with the prior art, the method provided by the invention can be used to prepare the chiral N-tert-butyloxycarboryl-3-hydroxypiperidine with the optical purity of 99% by using the racemic 3-hydroxypiperidine as a raw material, in addition, the material is cheap and easy to get, the reaction condition is mild, the operation is simple and feasible, the production cost is greatly lowered, and great industrial application value is achieved.
Owner:SYNCOZYMES SHANGHAI
Preparation method of supercritical-carbon-dioxide thickening agent
ActiveCN107253922AWell mixedUrea derivatives preparationCarbamic acid derivatives preparationRecovery methodCarbamate
The invention relates to a preparation method of a supercritical-carbon-dioxide thickening agent used for unconventional oil and gas reservoir development and aims to solve the problem that a conventional recovery method harms oil and gas reservoirs. According to the technical scheme, the method includes: adding deionized water into a bottle, adding ethanolamine, dropwise adding di-tert-butyl dicarbonate at 35 DEG C, performing reaction at 35 DEG C for 4 hours, and distilling to obtain an intermediate product (1) hydroxyethyl tert-butyl carbamate; adding the intermediate product (1) into a three-necked bottle, heating to 75 DEG C, dropwise adding perfluorooctanoyl fluoride, and performing reaction at 75 DEG C for 12 hours to obtain an intermediate product (2) ethyl perfluorooctanoate tert-butyl carbamate; adding dichloromethane and trifluoroacetic acid into the bottle, adding the intermediate product (2), performing reaction at 25 DEG C for 2 hours, performing extraction and vacuum drying, and adding 1, 6-hexamethylene diisocyanate to perform reaction for 10 minutes to obtain a final product (1, 6-diethyl perfluorooctanoate carbamido) hexane. The thickening agent can increase the viscosity of supercritical carbon dioxide and can be used for fracturing reformation and chemical reservoir oil displacement.
Owner:SOUTHWEST PETROLEUM UNIV
Synthesis method for GHK tripeptide
ActiveCN103665102AImprove lipophilicityAids in separation and purificationPeptide preparation methodsDipeptideSynthesis methods
The invention discloses a synthesis method for GHK tripeptide. A conventional liquid-phase synthesis method is adopted. The method comprises the following steps of protecting lysine by using p-nitrophenyl acetate, protecting dipeptide obtained by enabling Boc-Gly-OH to react with histidine by using di-tert-butyl dicarbonate, and enabling the protected dipeptide to react with the protected lysine to obtain the GHK tripeptide. According to the method, the p-nitrophenyl acetate is used for protecting the lysine, and can selectively acylate amino groups farther away from carboxylic acid functional groups, and compared with a conventional protecting method, the p-nitrophenyl acetate protecting method has the advantages that the operating steps are simple, and the production cost is lowered; the di-tert-butyl dicarbonate is used for protecting the dipepetide, so that the generation of other byproducts is reduced, the lipophicity of a chemical compound is improved, the separation and purification of a product are facilitated, the yield of the product is improved, and meanwhile, the generation of a recemization product can also be reduced.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Technique for synthesizing antineoplastic melphalan
InactiveCN101100440AStable in natureFast reduction reactionOrganic compound preparationAmino-carboxyl compound preparationAlcoholEsterification reaction
Synthesis of antineoplastic Melphalan is carried out by esterification reacting, amino-protection reacting, hydrogenation reducing, hydroxyethylation reacting, chlorination reacting, de-protection reacting, taking chlorinating agent, absolute alcohol protecting carboxyl and pyrocarbonic di-tert-butyl protecting amino, hydrolyzing by hydrochloric acid to obtain final product. It's cheap, efficient and non-toxic, has gentle reactive condition and can be used for industrial production. It's cheap, convenient, efficient and non-toxic and can be used for industrial production.
Owner:SUZHOU LEADER CHEM
Process for preparing dipeptidyl peptidase IV inhibitors and intermediates therefor
InactiveUS20050260712A1Procedure of process can be improvedReduce processing timeSugar derivativesBacteriaPhenylalanine dehydrogenaseDipeptidyl peptidase
A process for production of cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV is provided which employs a BOC-protected amine of the structure prepared by subjecting an acid of the structure to reduce amination by treating the acid with ammonium formate, nicotinamide adenine dinucleotide, dithiothreitol and partially purified phenylalanine dehydrogenase / formate dehydrogenase enzyme concentrate (PDH / FDH) and without isolating treating the resulting amine of the structure 2 with di-tert-butyl dicarbonate to form the BOC-protected amine.
Owner:ASTRAZENECA AB
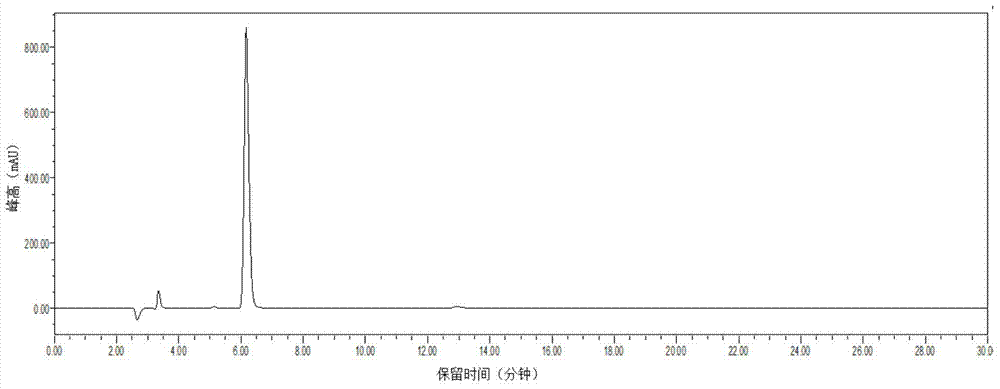
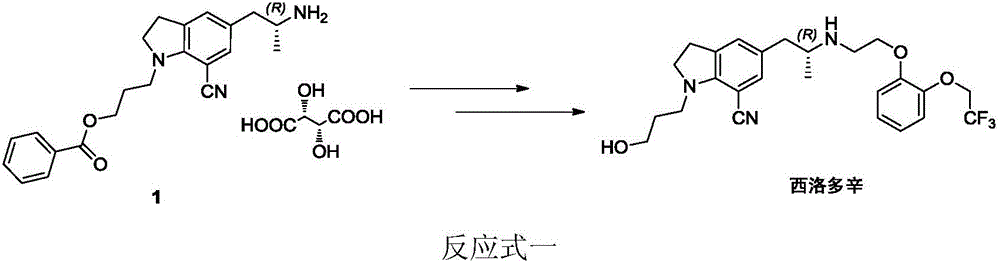
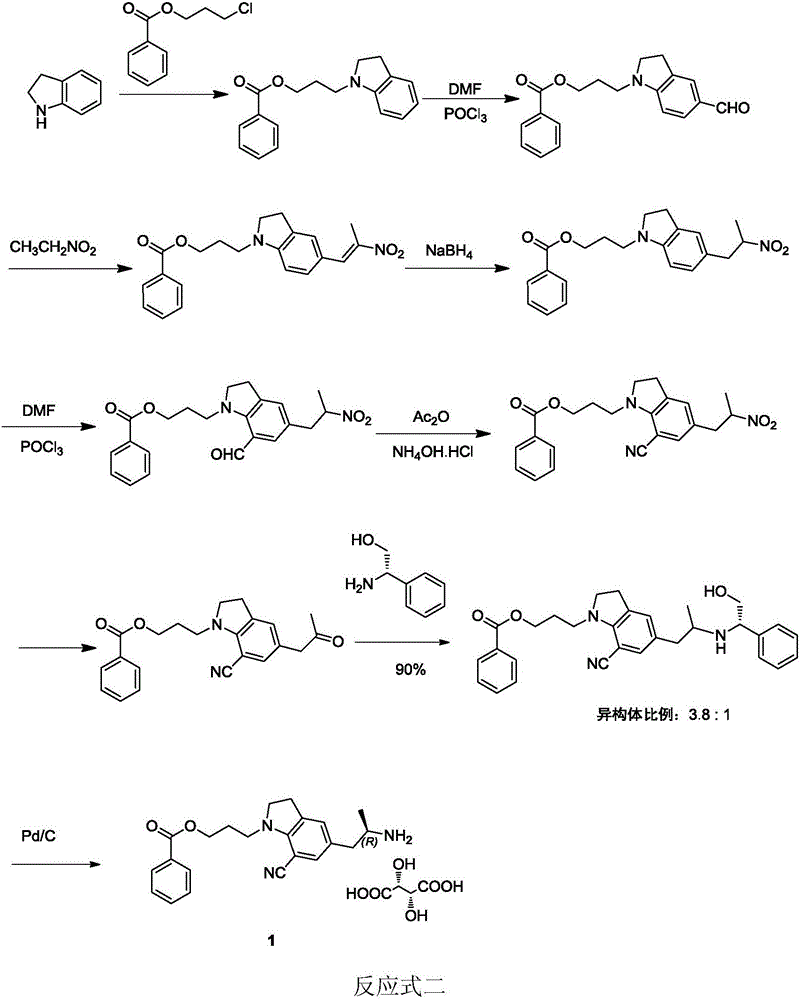
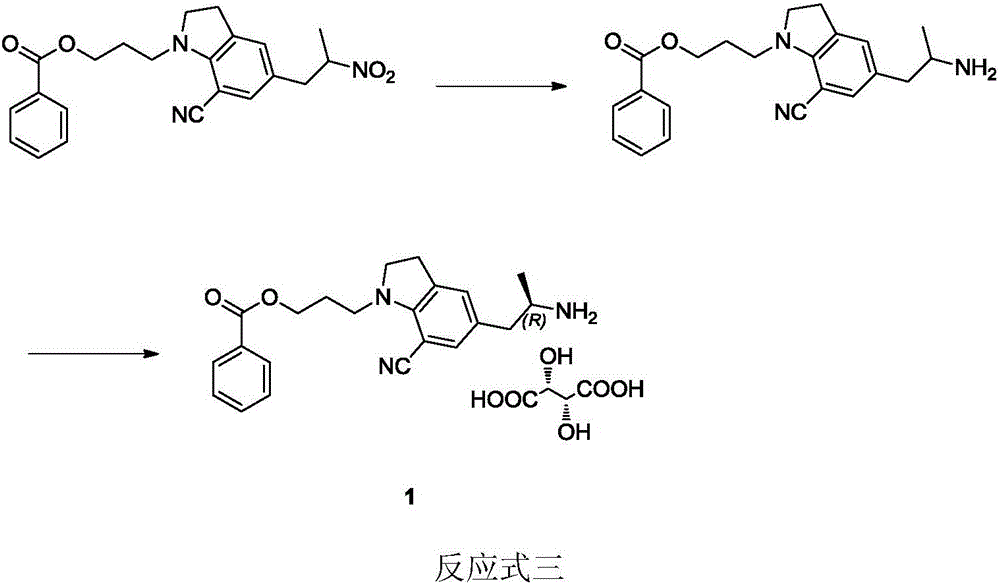
Preparation method of silodosin intermediate
The invention discloses a preparation method of a silodosin intermediate. The preparation method comprises the following steps that in an organic solvent, under the action of lewis acid, friedel-crafts acylation reaction occurs between a compound 2 and a compound 3, so as to obtain a compound 4, wherein the lewis acid is one of or more of zinc trifluoromethanesulfonate, bismuth trifluoromethanesulfonate, scandium trifluoromethanesulfonate and aluminum trichloride; under the action of organic acid or boron trifluoride ether complex, the compound 4 reacts with triethyl silicane, so as to obtain a compound 5; the compound 5 reacts with sodium azide, so as to obtain a compound 6; under the action of catalysts, the compound 6 reacts with di-tert-butyl dicarbonate ester and hydrogen, so as to obtain a compound 7; under an acidic condition, the deamination protective reaction of the compound 7 occurs, so as to obtain a compound 8; the compound 8 reacts with L-tartaric acid, so as to obtain a compound 1, namely the silodosin intermediate. The preparation method of the silodosin intermediate has the advantages of simplicity, economy and mild reaction conditions, and chiral resolution is not needed.
Owner:ZHEJIANG TIANYU PHARMA
Process for preparing Boc protected amino acid by (Boc) O
InactiveCN1793110ACheap and easy to useHigh yieldOrganic compound preparationAmino-carboxyl compound preparationSolventMedicinal chemistry
The invention discloses a method that uses (Boc)2O to make amino acid of Boc protection. It uses acetone and water as solvent, under the existing of (Et3N), takes reaction to form amino acid of Boc protection. The invention has high yield, easy to produce and no pollution. The product has good homogeneity, high purity and low cost.
Owner:WUHAN UNIV
Synthesis method for N-Boc-3-piperidone
InactiveCN103204801AEasy to separate and purifyShort synthetic routeOrganic chemistryPalladium on carbonBenzoyl bromide
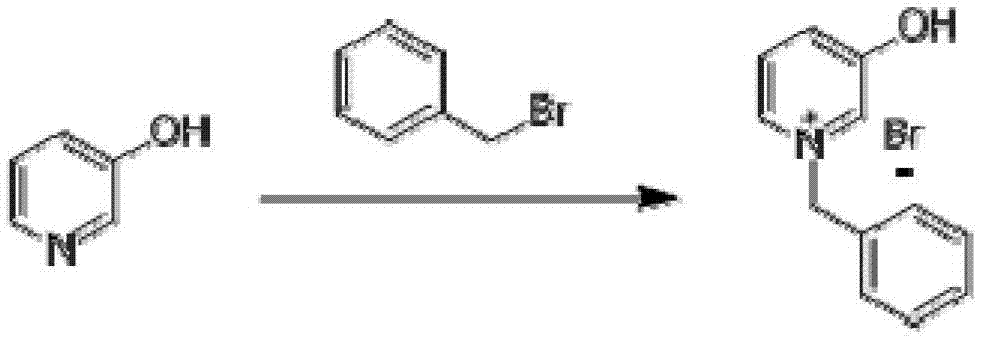
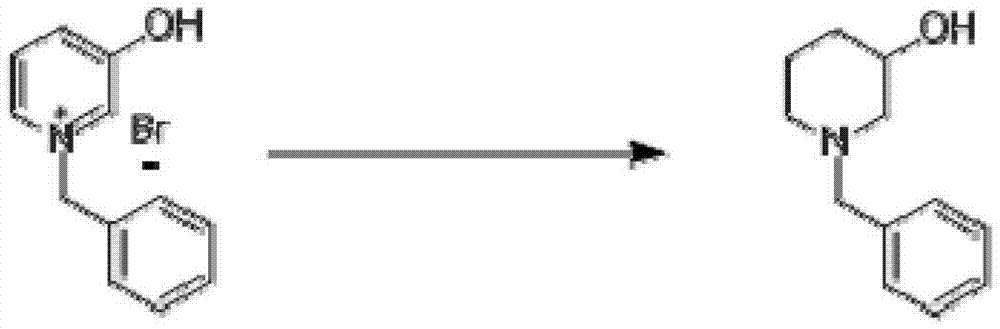
The invention discloses a synthesis method for N-Boc-3-piperidone. The synthesis method comprises the following steps of: reacting 3-hydroxyl pyridine with benzyl bromide in an organic solvent to obtain an N-benzyl-3-hydroxyl pyridine quaternary ammonium salt; reducing the N-benzyl-3-hydroxyl pyridine quaternary ammonium salt by sodium borohydride to obtain N-benzyl-3-hydroxyl piperidine; reacting N-benzyl-3-hydroxyl piperidine with di-tert-butyl dicarbonate ester to obtain N-Boc-3-hydroxyl piperidine under hydrogen protection and the catalysis of a palladium-carbon catalyst; and reacting N-Boc-3-hydroxyl piperidine with the mixed oxidant of dimethyl sulfoxide and oxalyl chloride to obtain N-Boc-3-piperidone under the action of an organic base. Compared with the existing synthesis method, the synthesis method disclosed by the invention is shorter in synthesis route, and easier for separation and purification of reactants, thus reducing the production cost, the energy consumption and the pollution; and the total productivity of N-Boc-3-piperidone can achieve more than 42%, and the purity thereof is greater than 98%.
Owner:甘肃天骄商贸有限公司
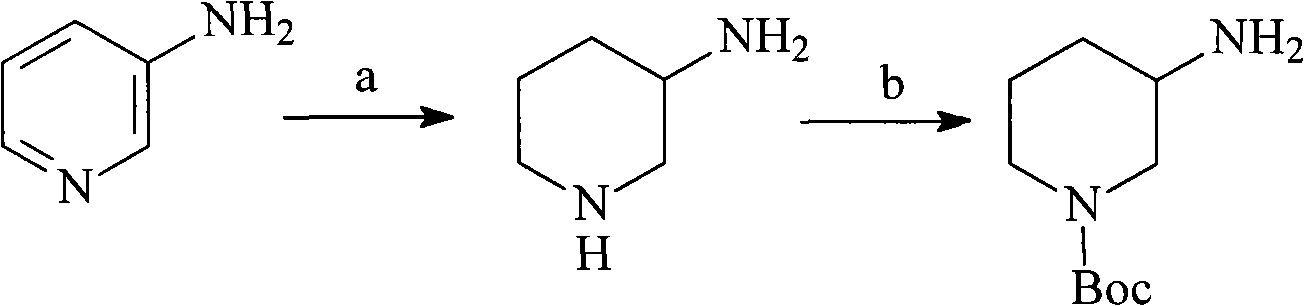
N-Boc-3-aminopiperidine and synthesizing method of optical isomer thereof
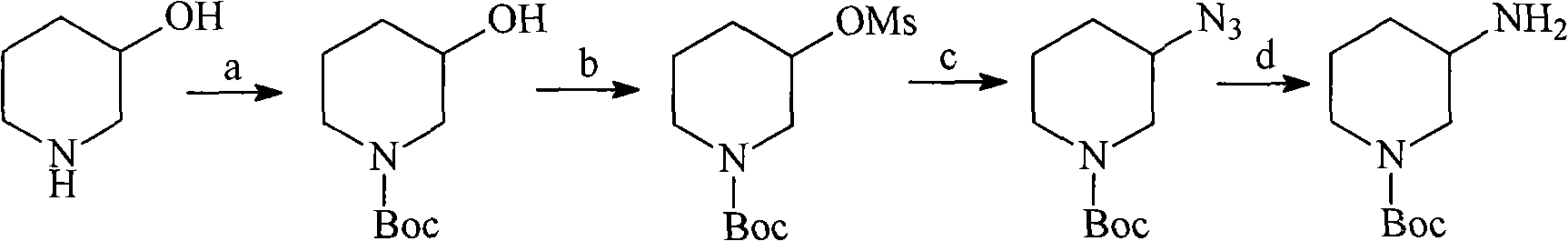
The invention discloses an intermediate N-Boc-3-aminopiperidine and a synthesizing method of optical isomer thereof. The prior synthesizing route has the disadvantages of high requirements on reaction conditions, high possibility of racemization, a great number of byproducts, as well as difficult post-treatment and difficult industrialization. The synthesizing method comprises the following steps: taking halogenated hydrocarbon as a solvent and organic alkali as an acid-binding agent, 3-piperidine ethyl formate is added with di-tert-butyl dicarbonate by dripping at temperature of 0-10 DEG C to obtain N-Boc-3-piperidine ethyl formate; 1,4-dioxane is used as a solvent, the N-Boc-3-piperidine ethyl formate undergoes ammonolysis reaction to obtain N-Boc-3-piperidine formamide; and the N-Boc-3-piperidine formamide is added by dripping in a solution with sodium hypochlorite and sodium hydroxide to obtain N-Boc-3-aminopiperidine. The invention has the advantages of no racemization during the reaction, high optical purity of the product, relatively moderate reaction conditions, simple operation, low total production cost, and easy large-scale industrial production.
Owner:NOVOCODEX BIOPHARMACEUTICALS CO LTD
Preparation method of glucose and temperature-responsive insulin controlled release carrier
InactiveCN103865013AWide variety of sourcesGlucose responsivePharmaceutical non-active ingredientsBiocompatibility TestingTrithiocarbonic acid
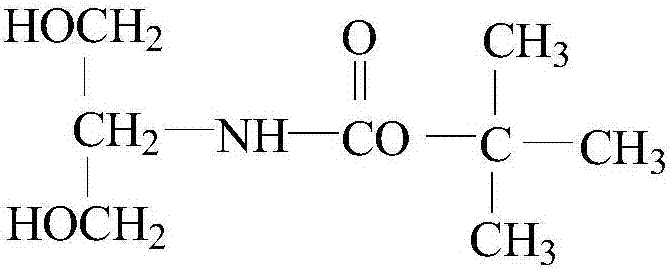
The invention relates to a preparation method of a glucose and temperature-responsive insulin controlled release carrier. 1,2-diaminoethane is used as an initial raw material, is protected through di-tert-butyl dicarbonate and then is reacted with acryloyl chloride to prepare 2-aminoethyl acrylamide, and then the 2-aminoethyl acrylamide is reacted with the reaction product 4-chloroformylphenylboronic acid pinacol ester of the 4-carboxybenzeneboronic acid to prepare the monomer N-acrylamide ethyl-4-(tetramethyl-dioxaborolan)-benzamide with glucose responsiveness. The Raft polymerization of the monomer is initiated through trithiocarbonate DDMAT, and then the obtained polymer is used as a macromolecular chain transfer agent to initiate the copolymerization of the MEO2MA and the OEGMA to obtain a block copolymer material PAEB-b-P. The block copolymer has glucose and temperature responsiveness, can control the release of the carried insulin according to the concentration of the glucose in the external environment, has biodegradability, biocompatibility and bioactivity and is widely applied in the fields of medicine controlled release carriers and biological intelligent switches. The preparation method is simple and easy, the raw material can be industrially produced, and the method has great popularization and application value.
Owner:TONGJI UNIV
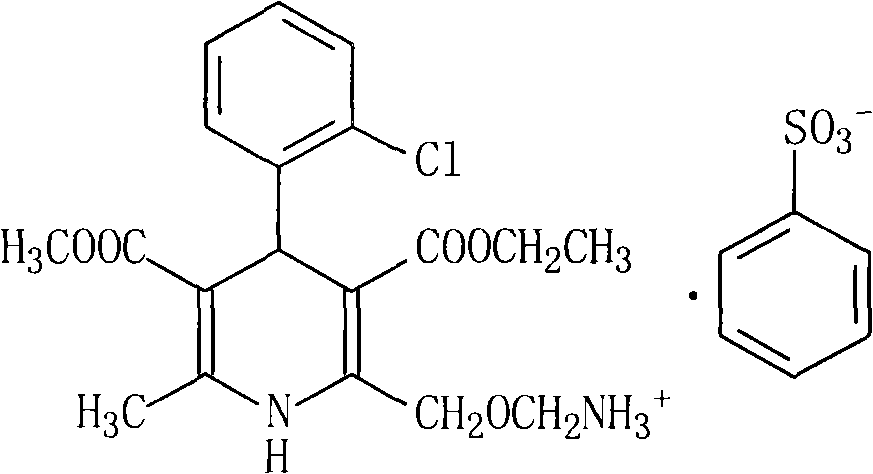
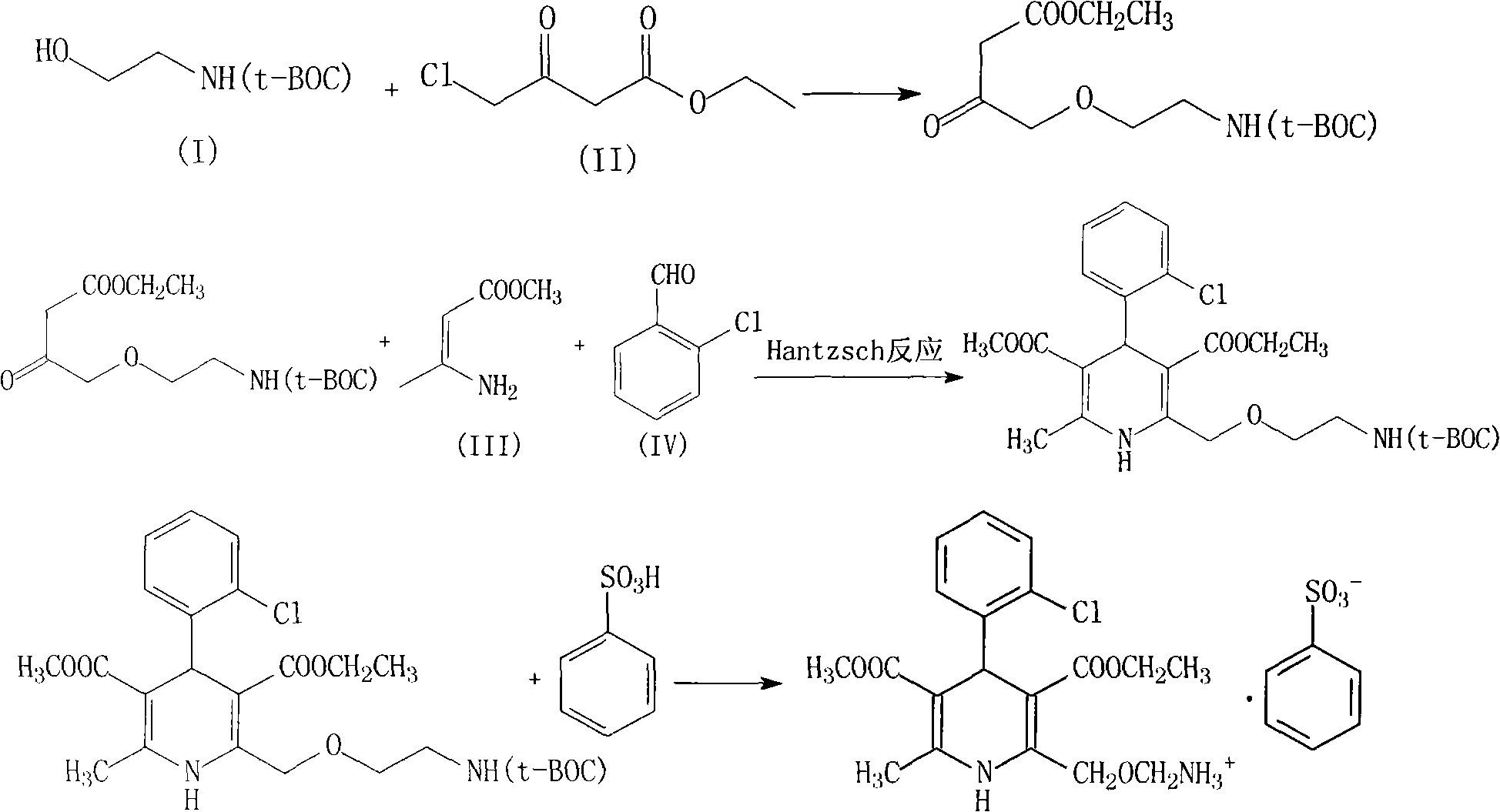
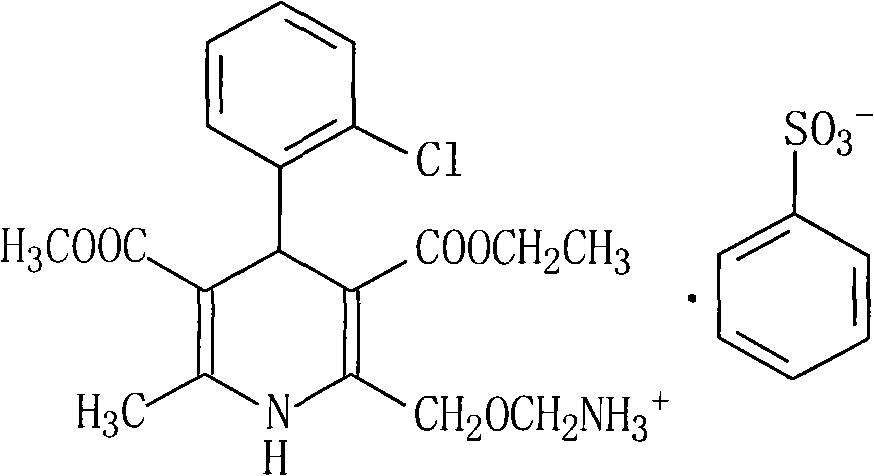
Amlodipine besylate compound and novel preparation method thereof
InactiveCN101812014ALow priceThe reaction steps are simpleOrganic chemistryBulk chemical productionCarbonic acidDi-tert-butyl dicarbonate
The invention relates to an amlodipine besylate compound and a novel preparation method thereof, wherein the method comprises the following steps that: a moderate amino-protecting reagent of di-tert-butyl dicarbonate is adopted so that the introduction of a Boc protecting group is realized, through the reaction of intermediums of 3-amino-crotonic acid methyl ester and 2-chlorobenzaldehyde, the obtained amlodipine besylate compound is directly carried out BOC protecting group removal reaction with benzene sulfonic acid, and the final product is obtained. Consequently, the invention is dispensed with the special deprotection step, simplifies the reaction steps, is more suitable for industrialized production and has high total reaction yield.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Preparation method of liquid carbon dioxide thickening agent
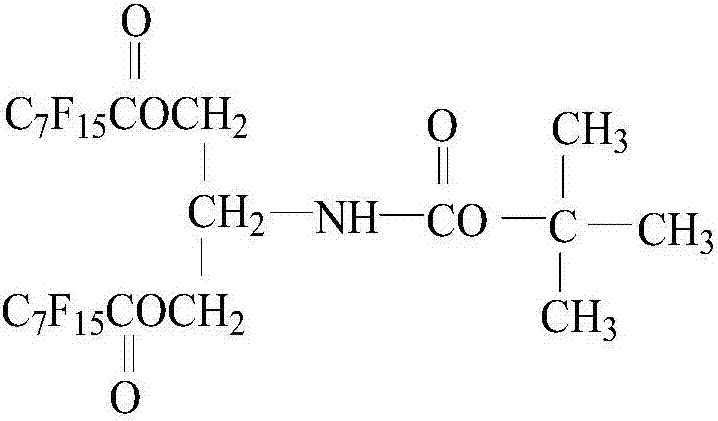
InactiveCN107043620ARelieve pressureImprove dissolutionUrea derivatives preparationOrganic compound preparationDistillationPerfluorooctanoic acid
The invention relates to a preparation method of a liquid carbon dioxide thickening agent for unconventional oil and gas reservoir development, and can solve the problem of damage to an oil and gas reservoir in a conventional mining mode. The preparation method has the technical scheme that methylbenzene and water are added into a bottle; then, serinol is added; terbutyloxycarbonyl anhydride is dropwise added at 0 DEG C; reaction is performed for 4h at 25 DEG C; distillation is performed to obtain an intermediate product (1); then, perfluoro caprylic acid is added into another bottle; thionyl chloride and N,N-dimethyl formamide are added at 75 DEG C; reaction is performed for 2h; distillation is performed to obtain an intermediate product (2); next, the intermediate product (1) is added into another bottle; the temperature is raised to 75 DEG C; the intermediate product (2) is dropwise added; reaction is performed for 12h at 75 DEG C to obtain an intermediate product (3); finally, dichloromethane and trifluoroacetic acid are added into the bottle; then, the intermediate product (3) is added; reaction is performed for 2h at 25 DEG C; after extraction and vacuum drying, hexamethylene diisocyanate is added to perform reaction for 2 minutes; a final product is obtained. The thickening agent can increase the carbon dioxide viscosity, and can be used for fracturing reformation.
Owner:SOUTHWEST PETROLEUM UNIV
Tyrosine phthalocyanines derivates, preparation thereof and applications in preparation of photodynamic drugs
InactiveCN101289450AHigh photosensitivityGood compatibilityOrganic active ingredientsGroup 3/13 element organic compoundsPhotosensitizerN dimethylformamide
The invention relates to a tyrosine phthalocyanine derivative which has a structural general formula as the right, in the formula, M is equal to Zn or Al, R is equal to H, Na or CH2CH3. The preparation method for the tyrosine phthalocyanine derivative is: (1) an intermediate II is obtained through the reaction of tyrosine and 4-nitrophthalic nitrile with protected amino group; (2) an intermediate III can be obtained by detracting Boc in the solution of dichloromethane and trifluoroacetic acid; (3) the tyrosine phthalocyanine derivative I can be obtained by cyclizing of the dinitriles intermediate that comprises the tyrosine; wherein, the Boc is di-tert-butyl dicarbonate, a DMF is N, N-dimethylformamide, and a DBU is 1,8-diazacyclo(5,4,0)hendecene-7. The invention also comprises the application of the tyrosine phthalocyanine photosensitizers in preparing photodynamic medicines.
Owner:NANJING NORMAL UNIVERSITY
Filgotinib synthetic method
ActiveCN104987333AWon't happenLess impuritiesOrganic chemistryChemical synthesisTert-Butyloxycarbonyl protecting group
The invention discloses a filgotinib synthetic method and belongs to the technical field of chemical synthesis of medicines. 6-chloro-2-aminopyridine and di-tert-butyl dicarbonate ester are subjected to condensation reaction to obtain 6-chloro-2-tert-butyloxycarbonyl aminopyridine; hydrolysis reaction is performed; the obtained 6-chloro-2-tert-butyloxycarbonyl aminopyridine and trifluorinated methyl sulfonic anhydride are subjected to trifluoromethanesulfonic acid esterification reaction; the obtained 2-tert-butyloxycarbonylamino-6-pyridyltrifluoromethanesulfonate and [(1,1-dioxo-4-thiomorpholinyl)methyl]benzo-4-boronic acid pinacol ester are subjected to condensation reaction to obtain a tert-butyl ester derivative; the tert-butyl ester derivative is treated by trifluoroacetic acid and subjected to de-protection; the obtained intermediate and ethoxycarbonyl isothiocyanate are subjected to isothiocyanate reaction; the obtained intermediate and hydroxylamine hydrochloride are subjected to ring closing reaction; the obtained intermediate and cyclopropane carbonyl chloride are subjected to amidation reaction to obtain the finished product. Operation is simplified; reagents are available; the concept of environment friendliness and environment protection is embodied.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Method for preparing macroporous adsorbent resin special for separating valine
ActiveCN101721979AOvercome purityOvercome efficiencyOrganic compound preparationOther chemical processesCross-linkTert-leucine
The invention relates to a method for preparing macroporous adsorbent resin special for separating valine, and belongs to the technical field of chemical and biological product processing. In the method, the types and proportions of an aqueous phase matter, cross-linking agents, polar monomers and pore-foaming agents are controlled in the process of synthesizing the resin, so that the resin can be bonded with di-tert-butyl dicarbonate effectively, and the synthesized macroporous adsorbent resin can separate the valine, alanine and leucine; and simultaneously, the macroporous adsorbent resin adopts pure water as a mobile phase, so that the pollution of a separation process on environment can be lightened effectively. The resin synthesized by the method can be effectively used for separating valine fermentation liquor and removing impurities with similar properties such as the alanine and the leucine in the valine fermentation liquor, and overcomes the defects of low purity, low efficiency, severe pollution and the like which exist in the conventional process for separating and purifying the valine, so that the purity of products reaches over 99 percent, reaches medicinal standards, and has high recovery rate and production strength.
Owner:XINTAI JIAHE BIOTECH CO LTD
Production method of fasudil hydrochloride
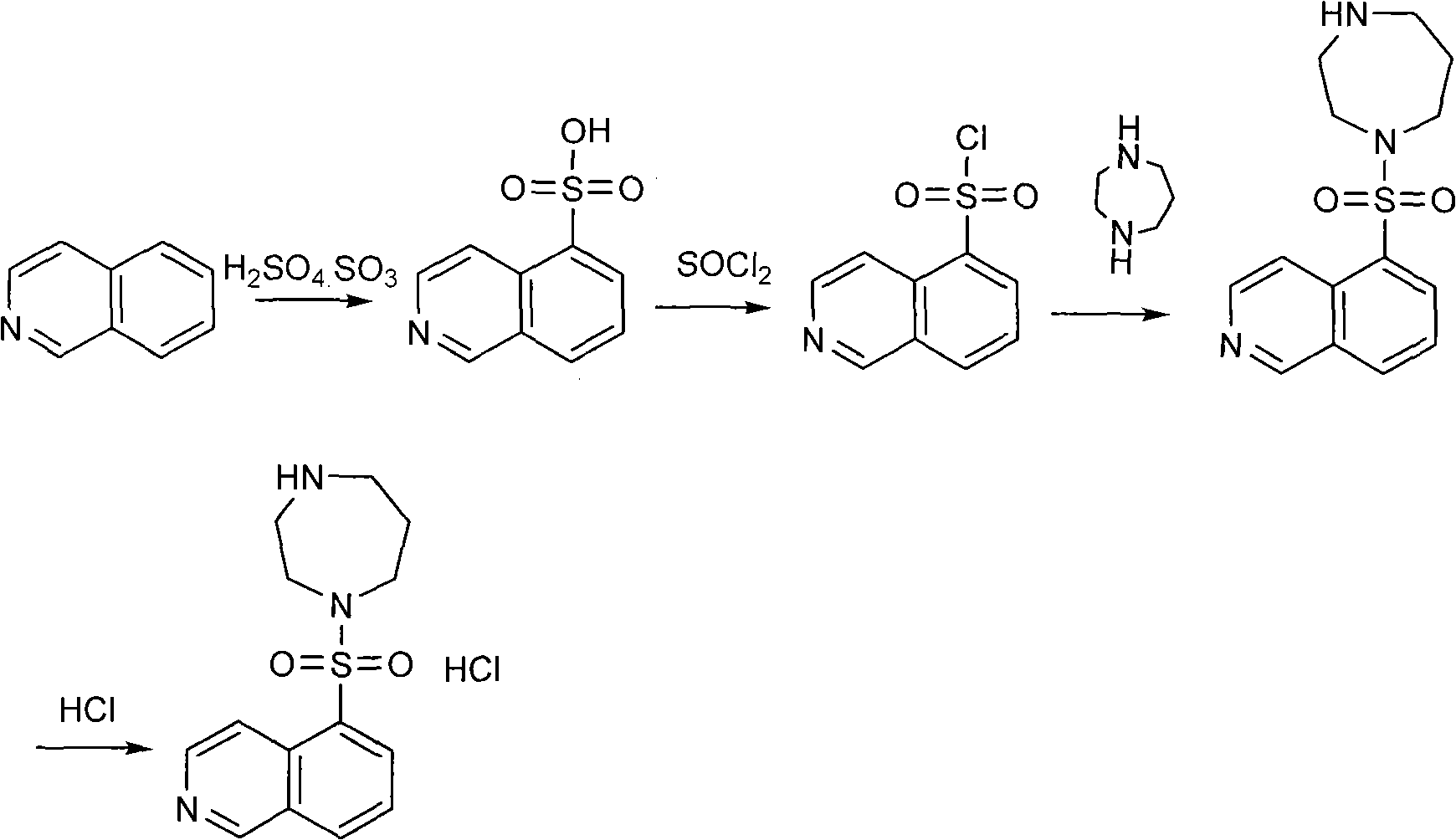
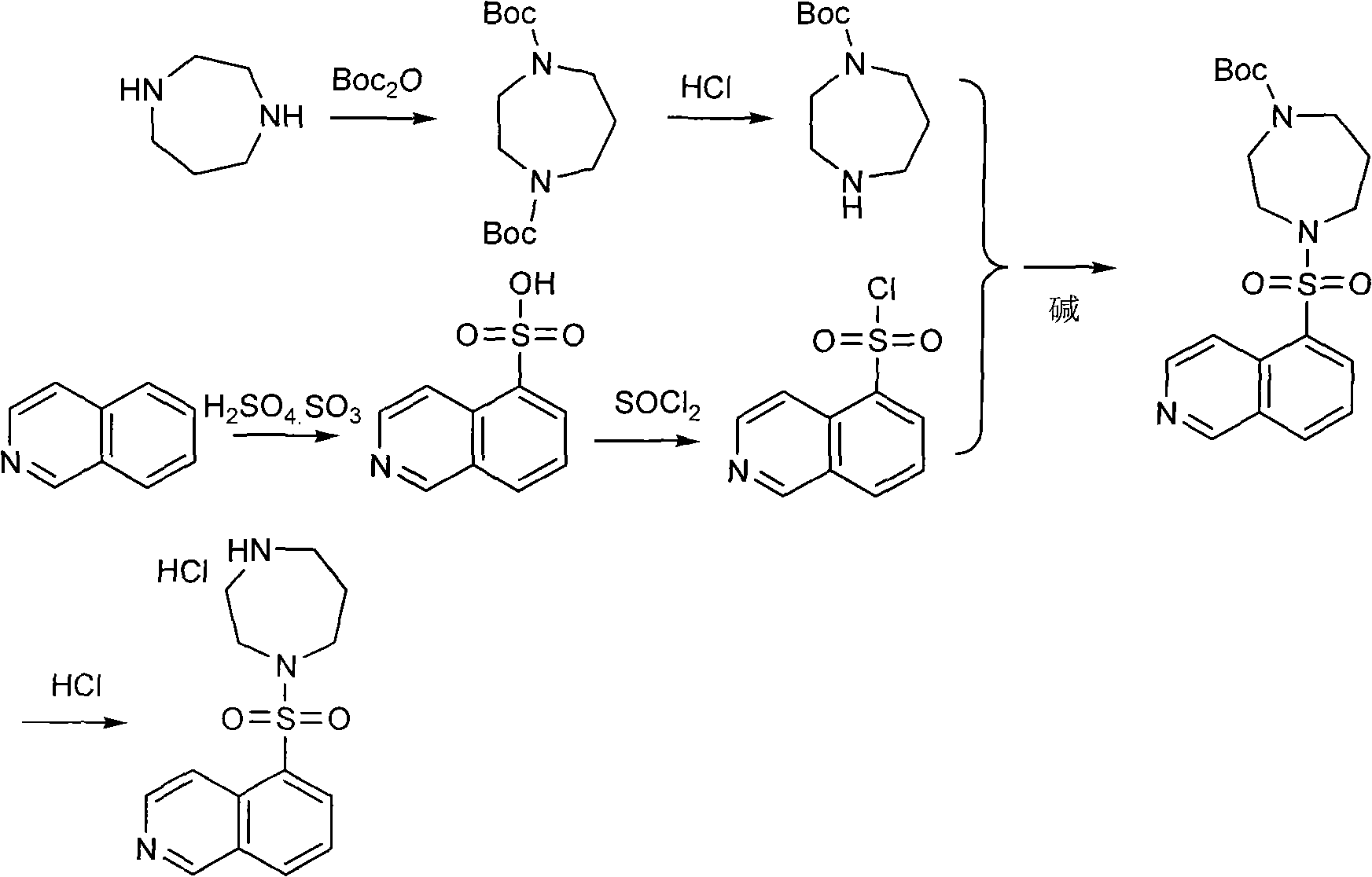
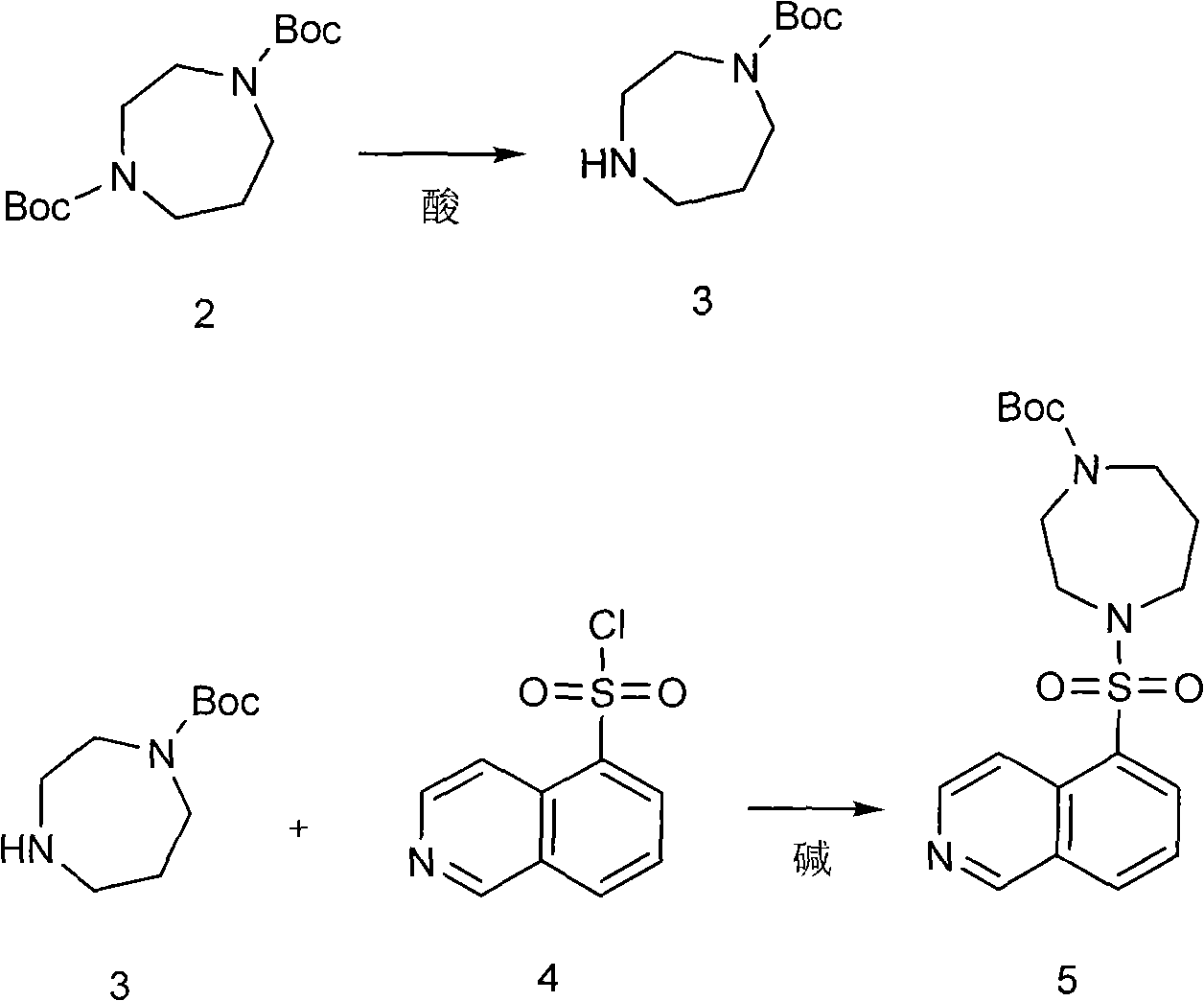
The invention discloses a production method of fasudil hydrochloride and relates to the field of medicament synthesis. According to the method, the traditional process is improved. The fasudil hydrochloride is prepared by protecting homopiperazine with di-tert-butyl dicarbonate to generate 1-Boc-homopiperazine and then reacting the 1-Boc-homopiperazine with isoquinolin-5-sulphonyl chloride. The improvement can greatly improve the reaction yield, simultaneously avoid the use of first kind or second kind of solvent and benefit the safety production and the industrial production.
Owner:河南省健康伟业生物医药研究股份有限公司
Rectification device
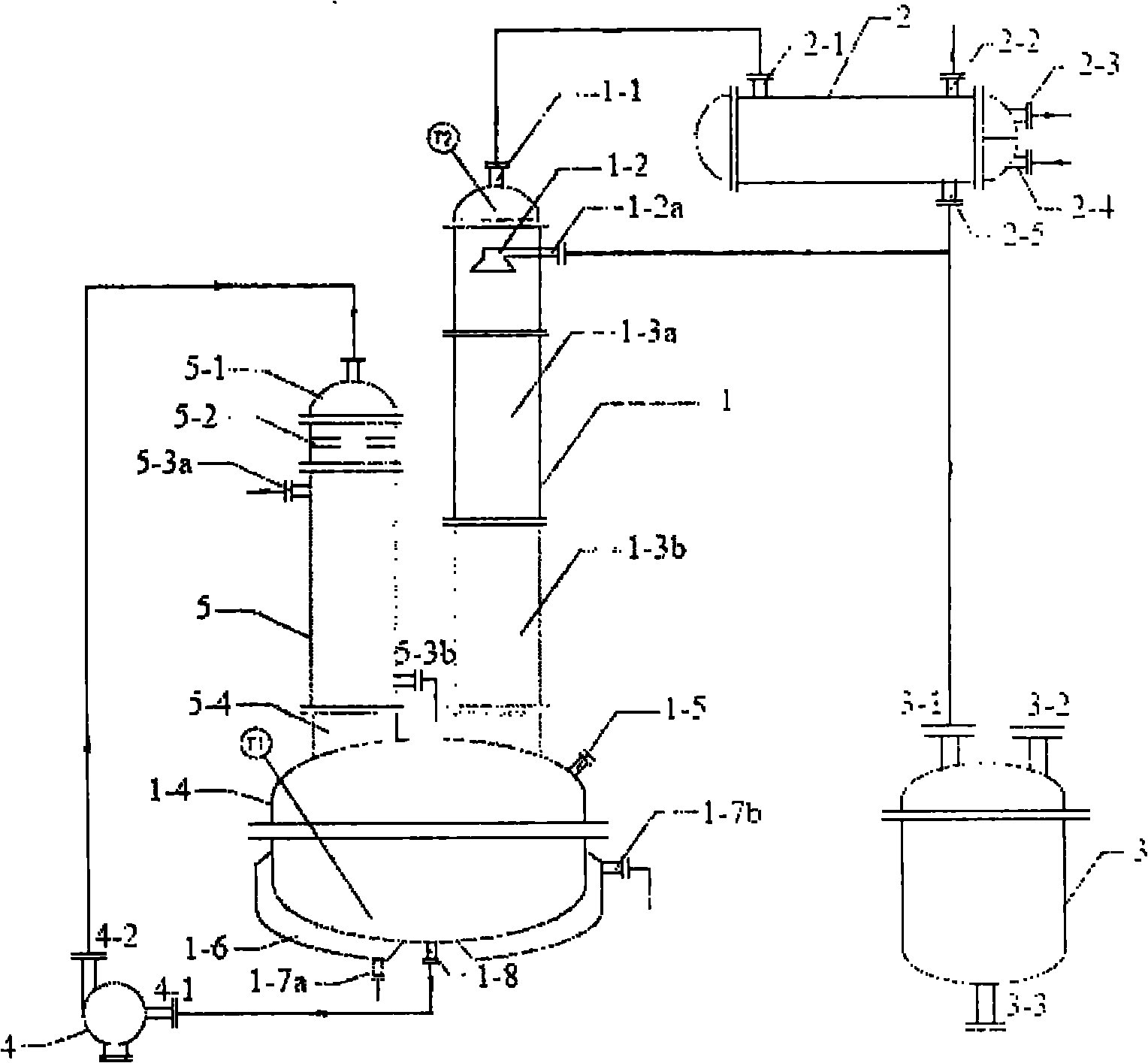
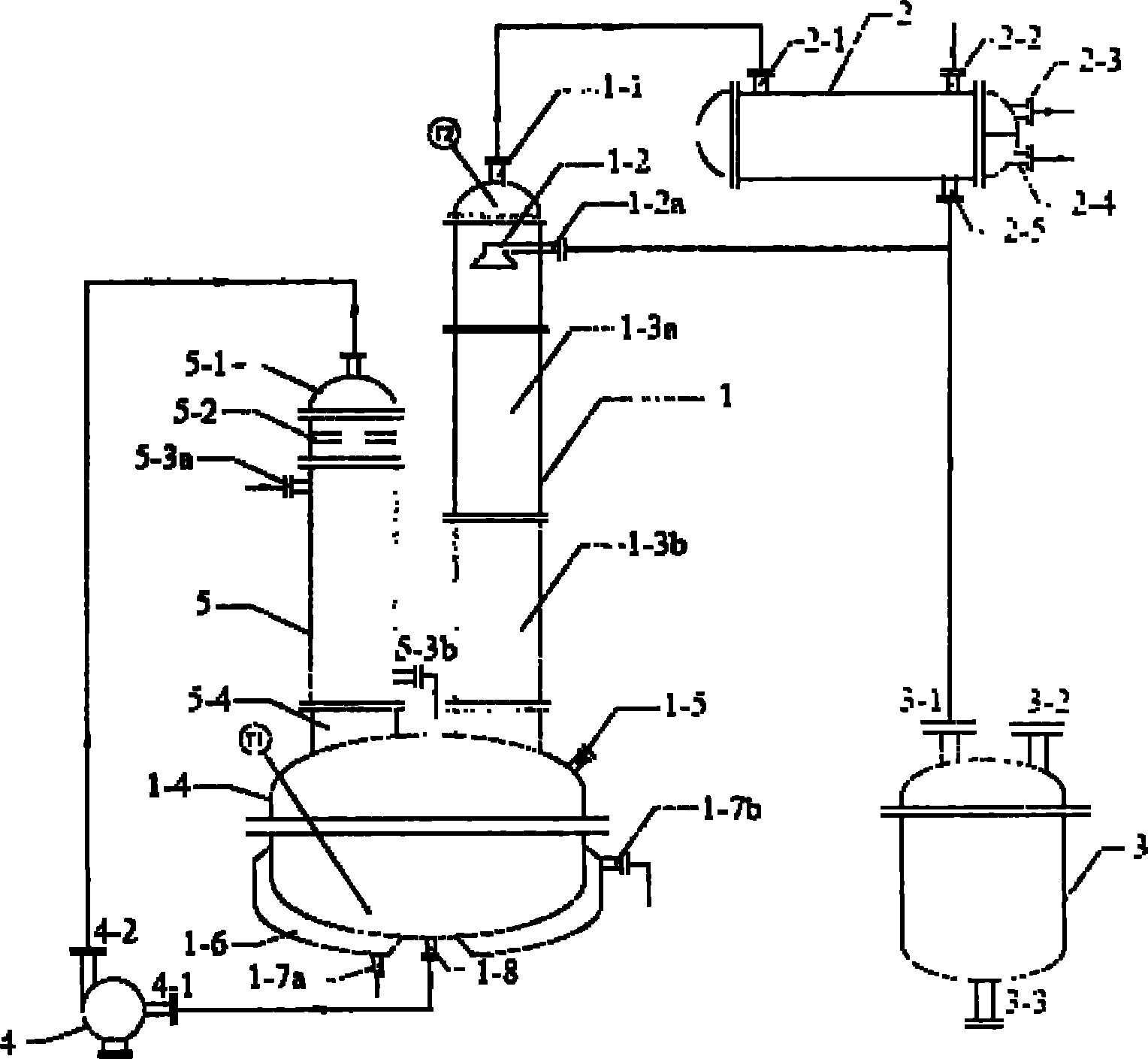
ActiveCN101961562AHigh recovery rateHigh purityDistillation separationPreparation from organic carbonatesReboilerCirculating pump
The invention belongs to chemical equipment, in particular to rectification equipment which is used for recovering butyl dicarbonate in crystallized mother liquor. The rectification device comprises a column kettle, a circulating pump, a falling film type reboiler, a pylon, a condenser and a receiving tank. The invention has the advantages of high recovery rate and high product purity of butyl dicarbonate, is especially suitable for recovering butyl dicarbonate in the crystallized mother liquor, improves work efficiency and lowers production cost.
Owner:GENCHEM & GENPHARM CHANGZHOU CO LTD
Amide arylpiperazine derivatives, their preparation method, and their application in benign prostatic hyperplasia resistance
InactiveCN103387531ALow antagonistic activityIncrease the lengthOrganic active ingredientsOrganic chemistryHydrobromideCarboxylic acid
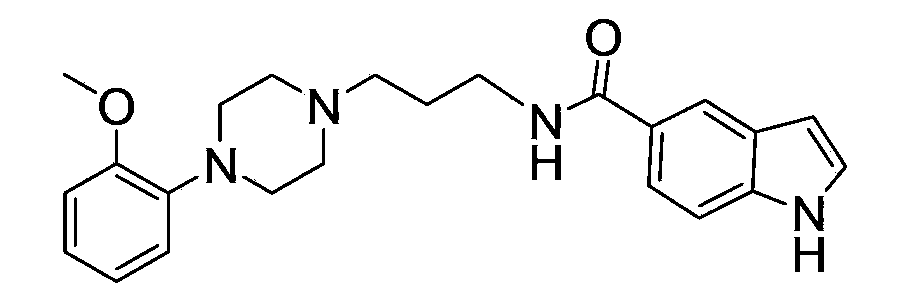
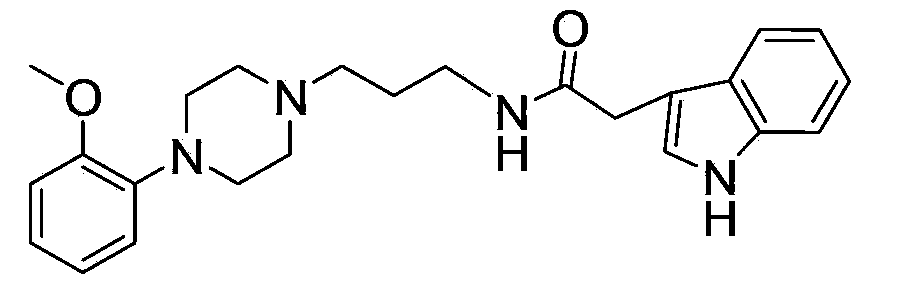
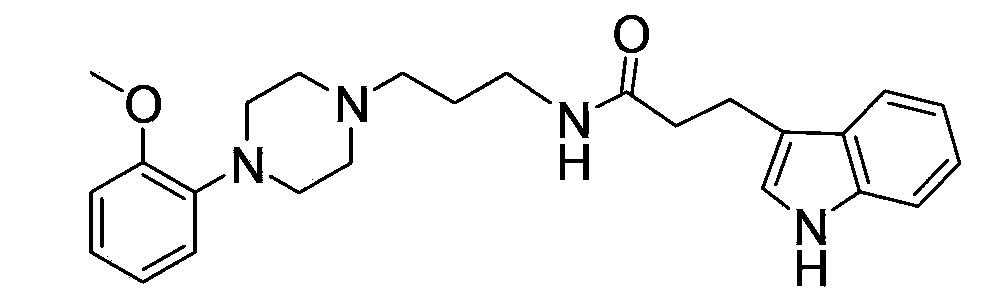
The invention relates to ten amide arylpiperazine derivatives, their preparation method, and their use in the preparation of benign prostatic hyperplasia treatment medicines. The preparation method of the amide arylpiperazine derivatives comprises the following steps: reacting free alkali of 3-bromopropylamine hydrobromide with di-tert-butyl dicarbonate to obtain a colorless oily liquid, reacting the colorless oily liquid with o-methoxyphenylpiperazine to obtain a light yellow oily material, reacting the light yellow oily material with trifluoroacetic acid to obtain a product, and reacting the product with N,N-diisopropylethylaine, 2-(7-azobenzotriazol)-N,N,N',N'-tetramethylformamidinium hexafluorophosphate, and 5-indoleacetic acid or 3-indoleacetic acid or 3-indolepropionic acid or 3-indolebutyric acid or 5-hydroxy-2-indoleacetic acid or 5-methoxy-2-indoleacetic acid or 1-methyl-3-indoleacetic acid or 6-bromo-2-indoleacetic acid or 5-chloro-2-indoleacetic acid or 7-nitro-2-indoleacetic acid to obtain final products. Experiments prove that the benign prostatic hyperplasia resistance activities of eight compounds in the amide arylpiperazine derivatives are stronger than a contrast drug prazosin.
Owner:广州医学院
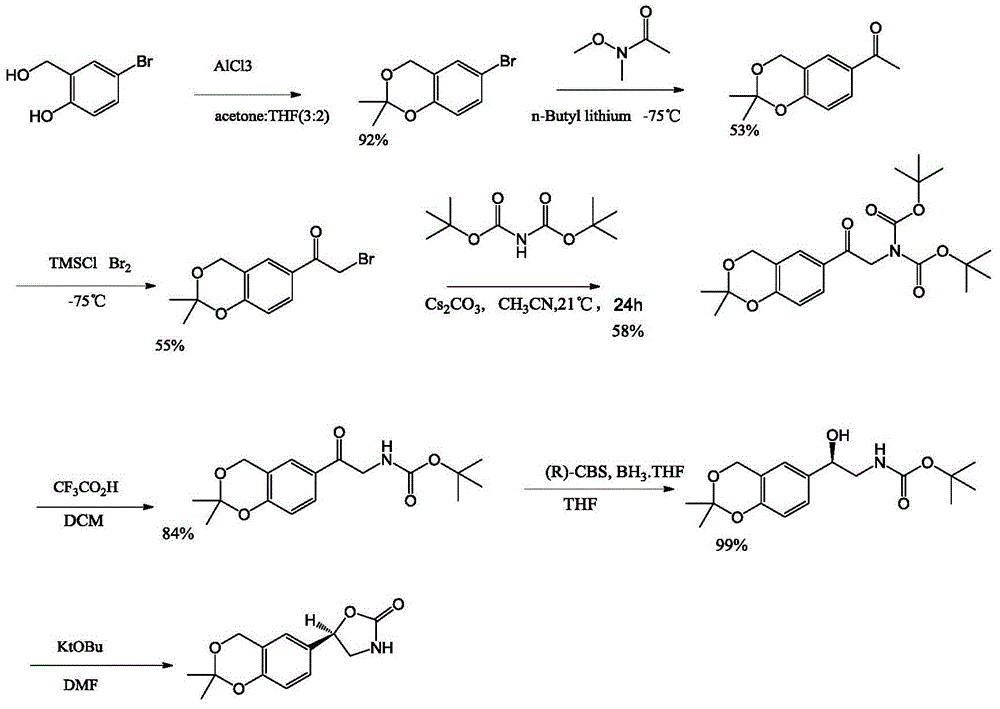
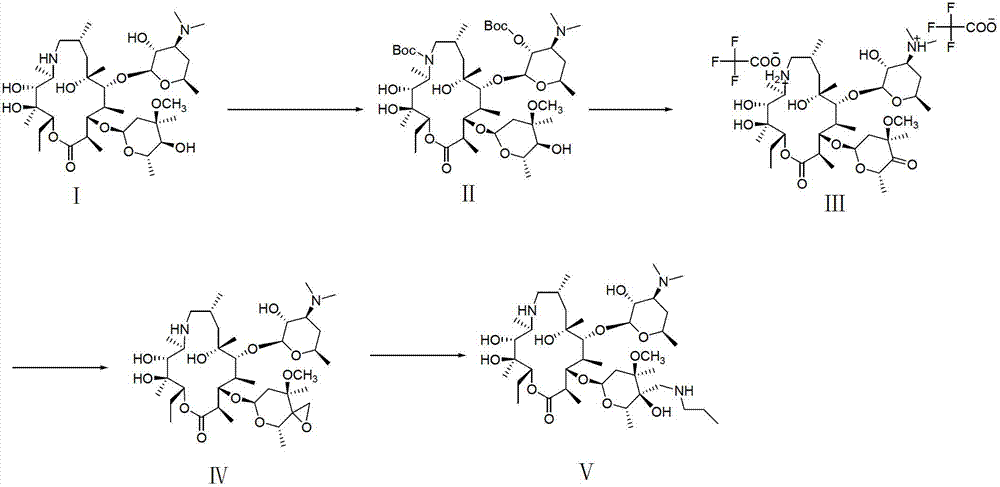
Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes
ActiveCN103420896APromote safe productionEasy to operateOrganic chemistryTert-Butyloxycarbonyl protecting groupKetone
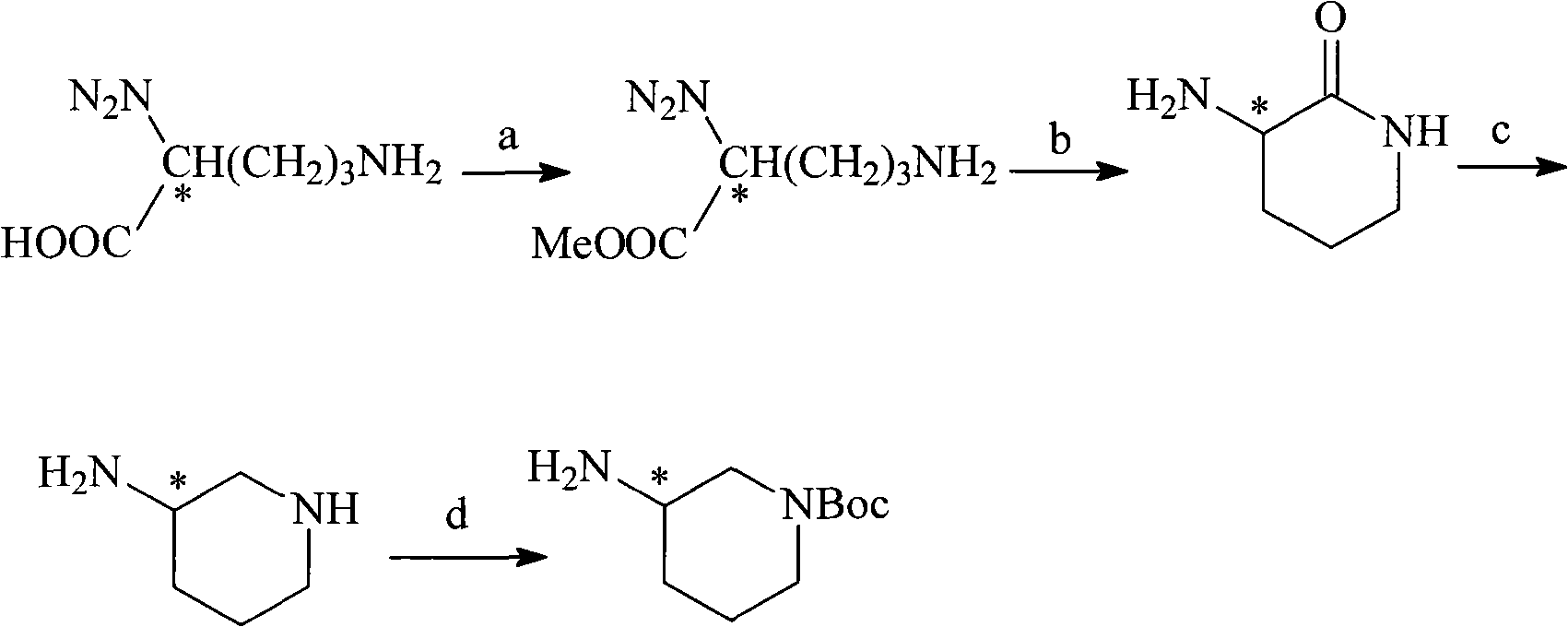
The invention discloses a preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptane. The method comprises the following steps: reacting benzoylamide acetoacetate as a raw material with 1,2-dichloroethane to obtain 3-cyclopropyl benzoylamide acetoacetate, brominating 3-cyclopropyl benzoylamide acetoacetate by NBS (n-bromosuccinimide) to obtain 1-bromo-3-cyclopropyl benzoylamide acetoacetate, cyclizing under alkaline conditions to obtain 5-benzyl-5-aza-spiro[2,4]heptane-4,7-diketone, further reacting with hydroxylamine hydrochloride to form an oxime compound-4-oxo-5-benzyl-7-oximido-5-aza-spiro[2,4]heptane, reducing by NaBH4 and boron trifluoride diethyl etherate to obtain 5-benzyl-7-amino-5-aza-spiro[2,4]heptane, performing chiral resolution by a resolving agent-L-camphorsulfonic acid to obtain 5-benzyl-7(S)-amino-5-aza-spiro[2,4]heptane, and reacting with di-tert-butyl dicarbonate ester to obtain 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptane. According to the method, an intermediate body-carbonyl does not need protection, raw materials are easy to get, a process route is simple, and the method is suitable for industrial production.
Owner:苏州楚凯药业有限公司
Method for preparing primary amine carbon nano tube
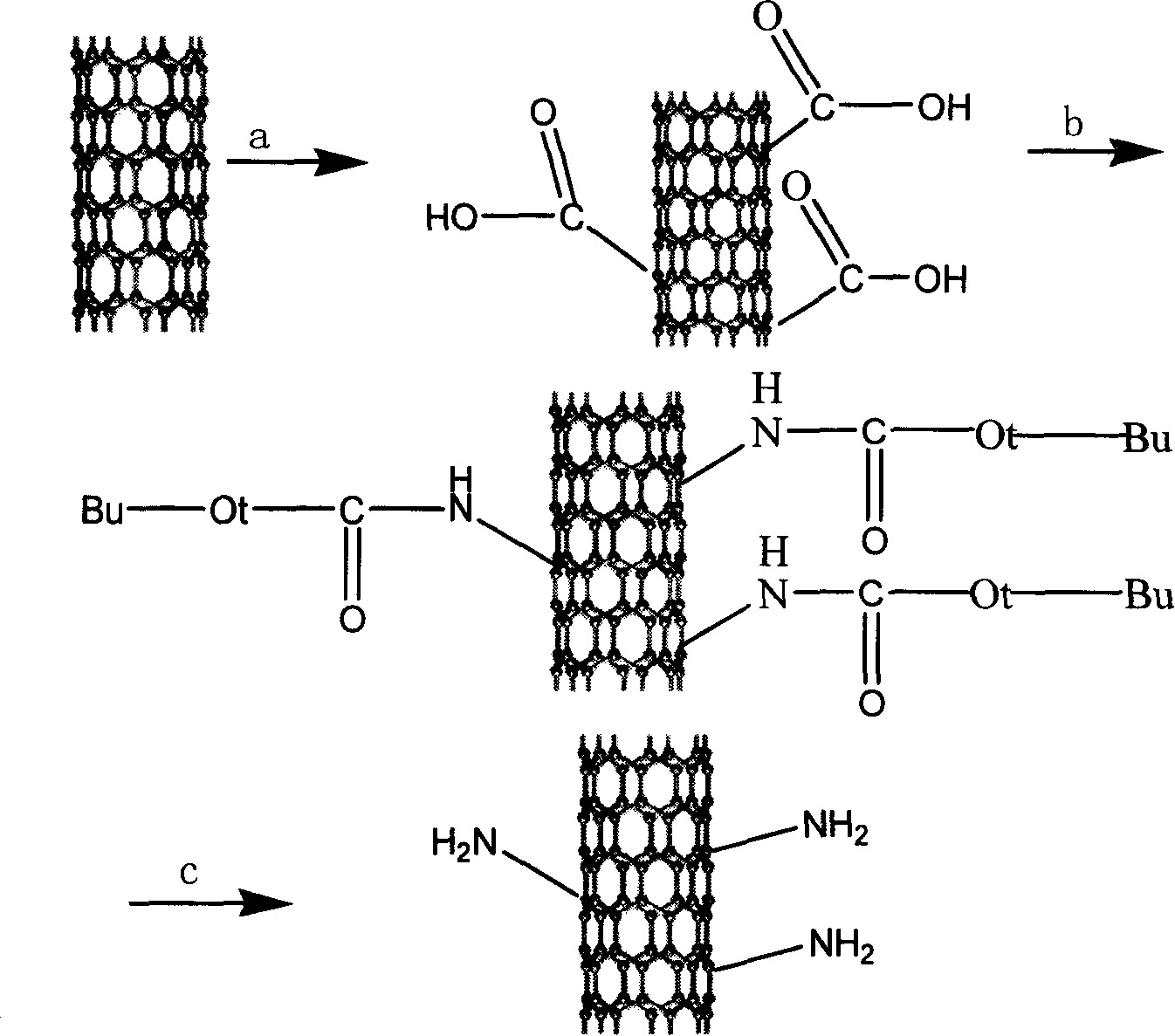
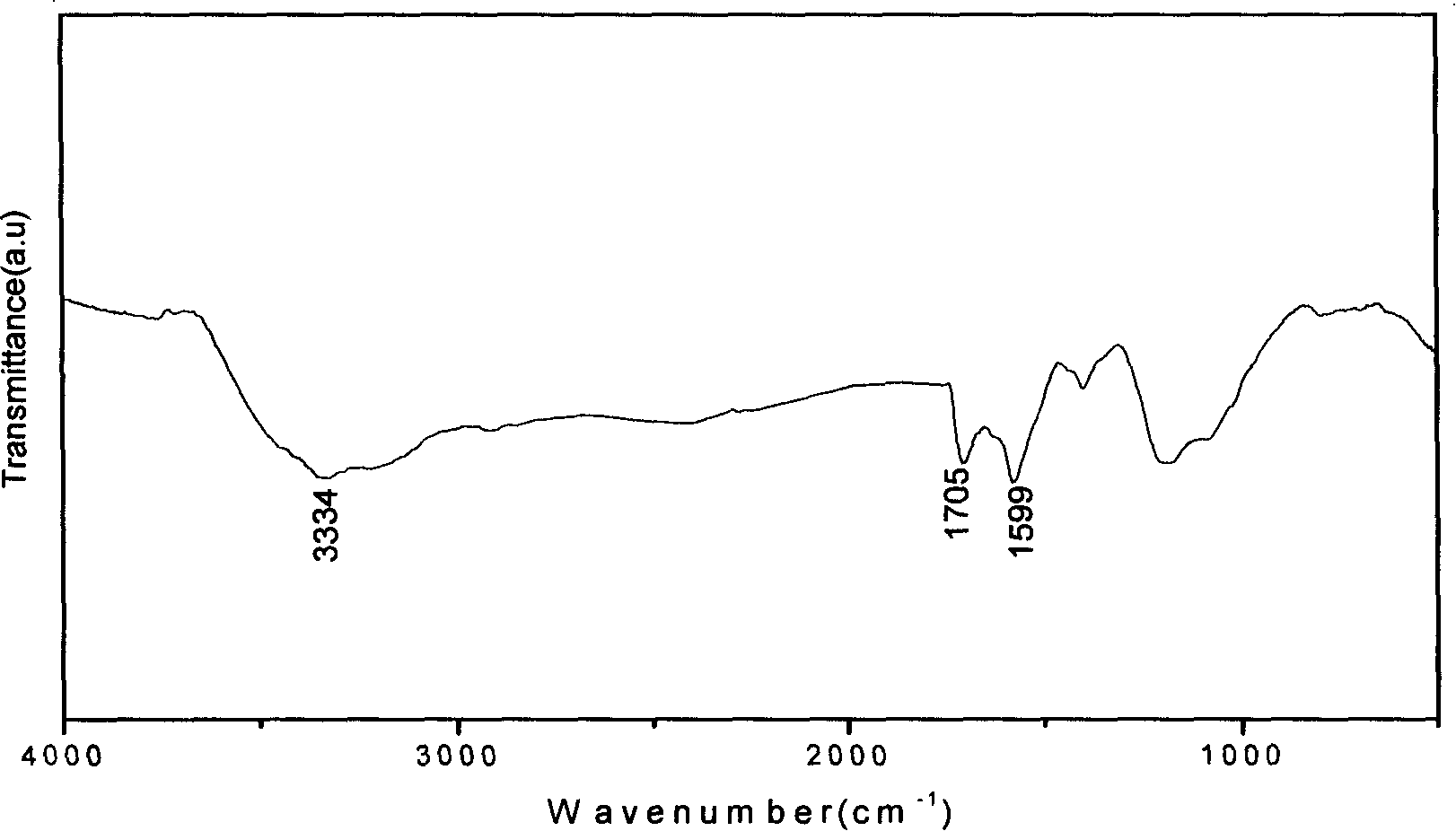
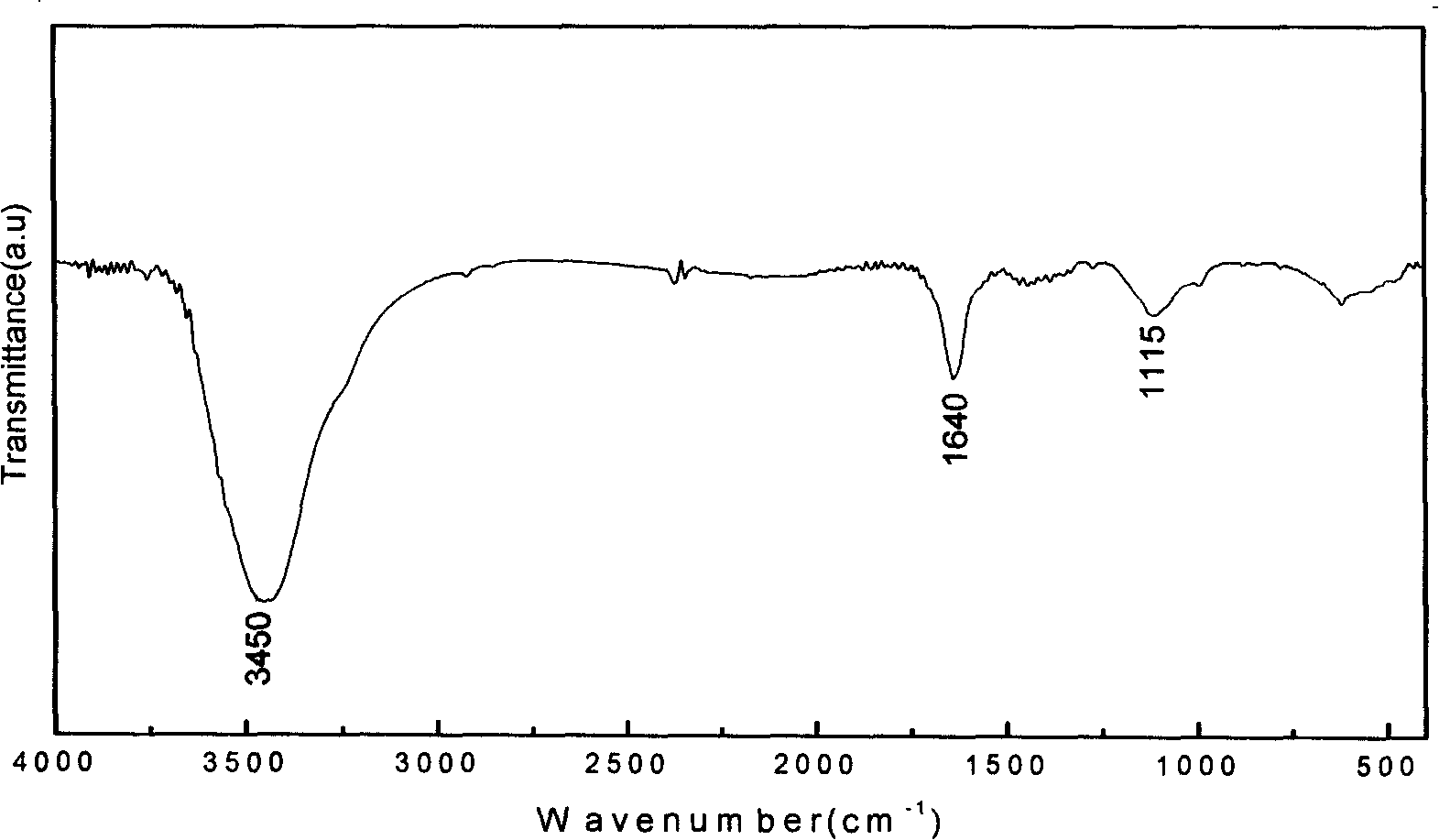
InactiveCN101177260ASpecial structureSpecial performanceNanostructure manufactureModified carbonReaction intermediate
The invention relates to a preparation method for primary amine modified carbon nano-tubes (CNT). Firstly, the carbon nano-tubes (CNT) with carboxyl at surface are obtained after acid treatment of the carbon nano-tubes (CNT). Carboxyl carbon nano-tubes (CNT), di-tert-butyl dicarbonate and sodium azide are reacted under the composite catalysis of phase-transfer metal catalyst and Lewis acid to obtain the carbon nano-tubes (CNT) with carbamic tert-butyl. Then the tert-butyl group is lost in hydrochloric ethyl acetate solution to generate the carbon nano-tubes (CNT) with primary amine group. Compared with other methods, the carbon nano-tubes (CNT) prepared with the invention has higher primary amine level, and can be used as an important reaction intermediate as well as be compounded with polymers to obtain composite materials with high performance.
Owner:TONGJI UNIV
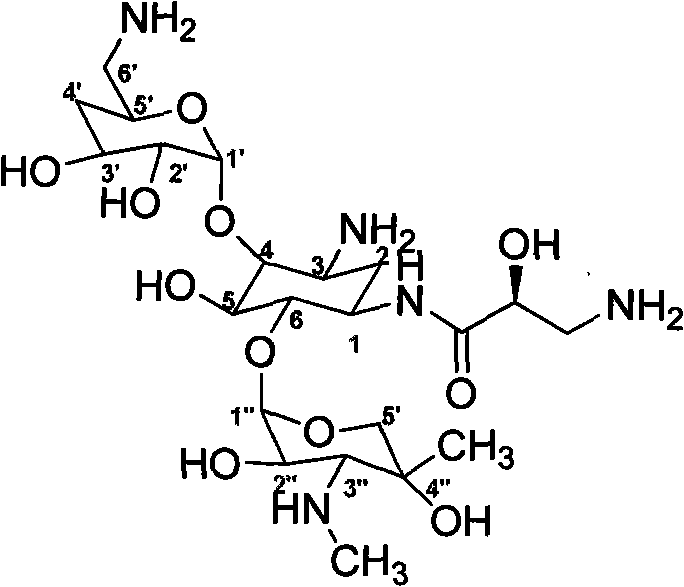
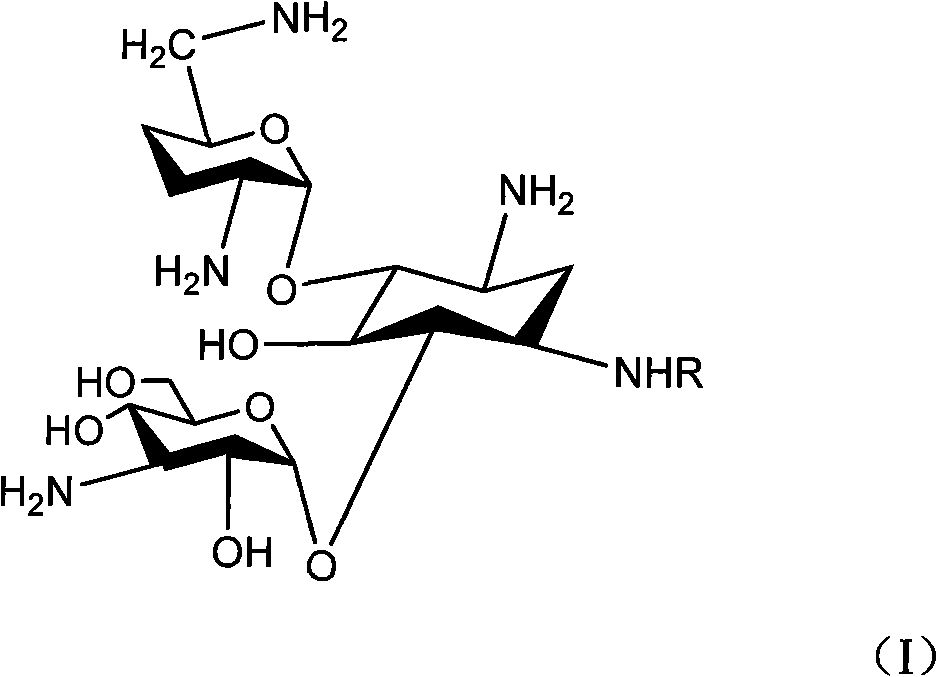
Method for preparing isepamicin and salts thereof
InactiveCN102093444AHigh selectivityHigh yieldSugar derivativesSugar derivatives preparationGentamicin BNitrogen
The invention relates to a method for preparing isepamicin for medicinal purpose, which comprises the following steps: (i) performing chelation reaction of gentamicin B and soluble inorganic zinc salt, and performing the amidation reaction of the product of the chelation reaction and butyl dicarbonate to form 3,6'-dibutyloxyacyl gentamicin B; (ii) performing the condensation reaction of the 3,6'-dibutyloxyacyl gentamicin B and nitrogen protected (S)-isoserine to form nitrogen protected isepamicin; and (iii) the removing the nitrogen protective group from the nitrogen protected isepamicin to form isepamicin. The invention also relates to a method for preparing isepamicin salts. The method of the invention has the advantages of high selectivity, high yield, low cost, simple operation, easy industrialization and the like.
Owner:江西制药有限责任公司
New synthetic method of PET imaging agent L-5-<18>FETP
InactiveCN101648899ASimple methodMethod stableOrganic chemistryRadioactive preparation carriersCarboxyl radicalEthylene glycol bis
The invention relates to a new synthetic method of a PET imaging agent L-5-<18>FETP. The method comprises the following steps: (a) carrying out a reaction between 5-hydroxytryptophan and C<1-3>alkylolto obtain a product; carrying out a reaction between the product and di-tert-butyl (i.e. (BOC)2O) to obtain N-BOC-L-5-hydroxytryptophan; (b) fluorizating 1,2-glycol tosylate in the presence of the ions such as potassium carbonate, Kryptate2.2.2 and <18>F<-> to obtain 2-<18F>-fluoroethanol p-toluene sulphonic acid ester (i.e. <18>FCH2CH2OTs); (c) carrying out a reaction between the 2-<18F>-fluoroethanol p-toluene sulphonic acid ester and the alkali metal salt of the N-BOC-L-5-hydroxytryptophan (such as sodium salt) to obtain O-(2-<18F>fluoroethyl)-N-BOC-L-5-hydroxytryptophan; (d) hydrolyzing the O-(2-<18F>fluoroethyl)-N-BOC-L-5-hydroxytryptophan under acidic conditions to remove an amino group and a carboxy protective group and obtain a final product of O-(2-<18F>fluoroethyl)-N-BOC-L-5-hydroxy tryptophan; and (e) purifying the product. The method greatly improves the radiochemical yield, obtains a sufficient quantity of a target product and realizes the clinical application of the target product.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
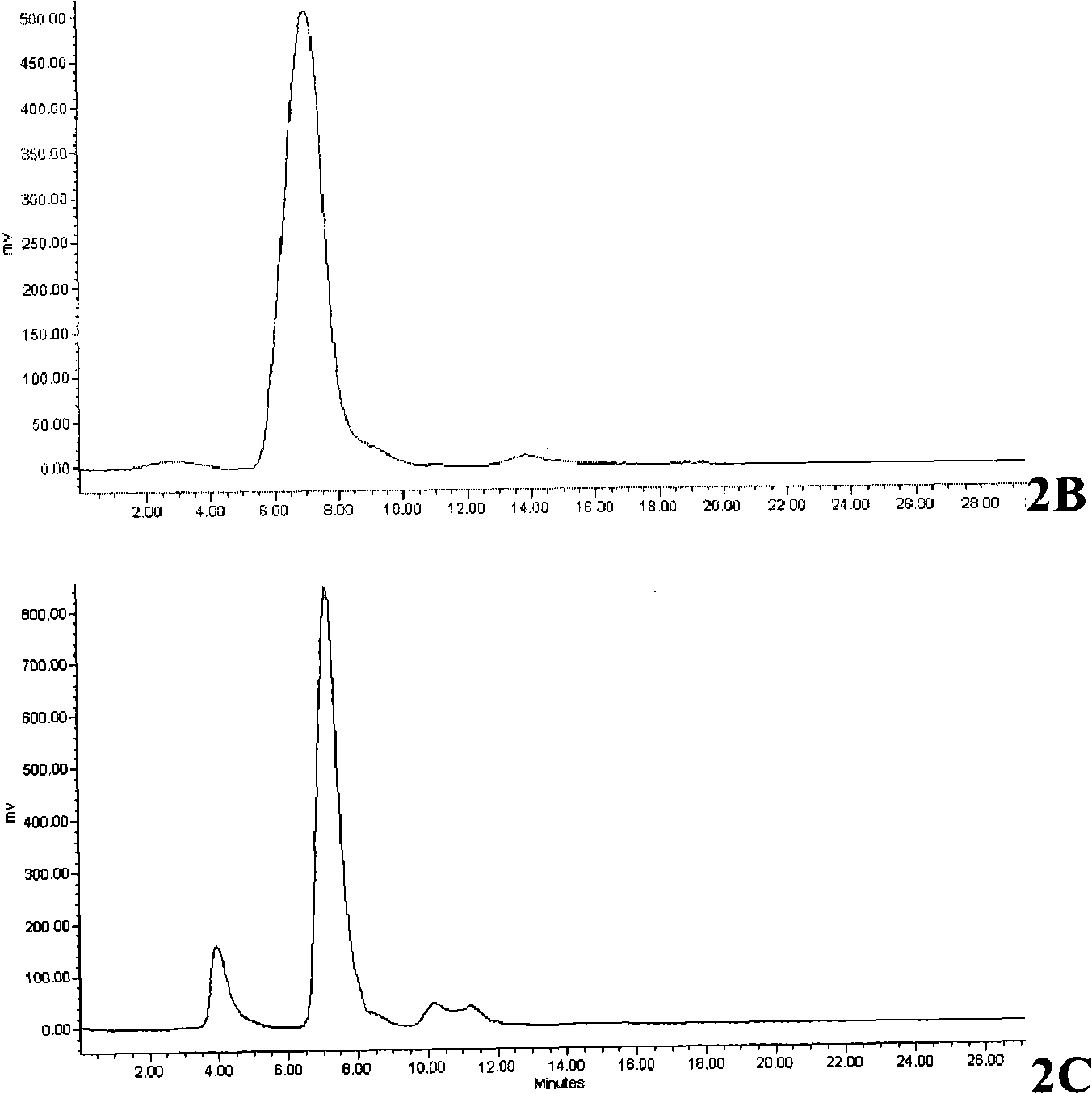
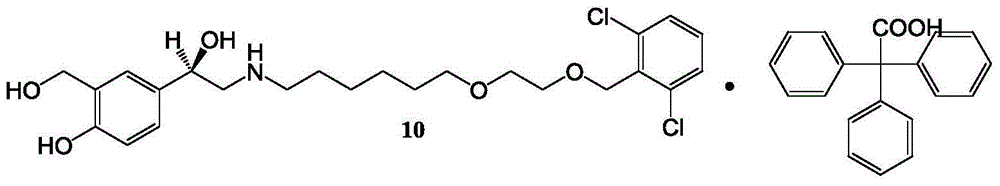
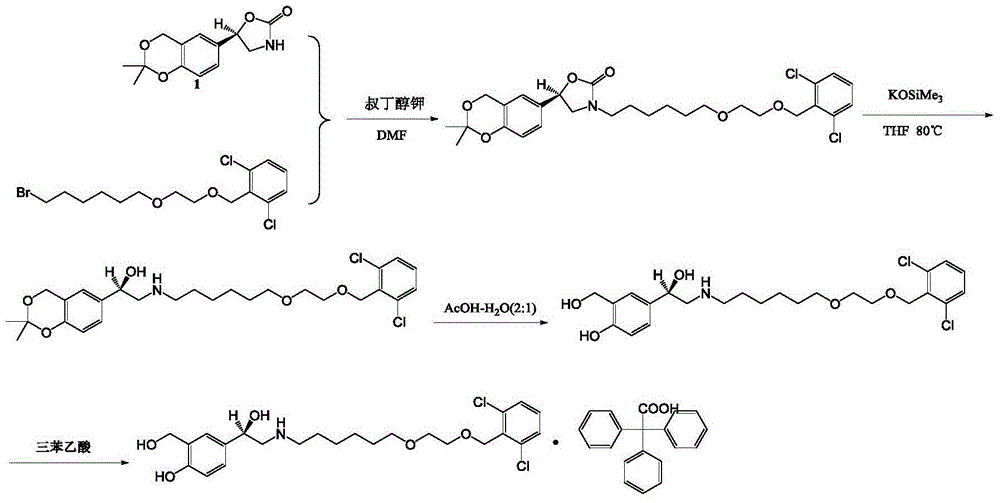
Vilanterol intermediate, preparation method and application thereof
ActiveCN105646285AHigh yieldReduce yieldCarbamic acid derivatives preparationOrganic compound preparationBenzeneCarboxylic acid
The invention provides a new Vilanterol intermediate compound 2. According to the invention, primary amino is introduced into a compound 5 through Delepine reaction, then di-tert-butyl dicarbonate is employed for amino protection, and the strategy greatly improves the yield and atom utilization rate. On the other hand, cheap and easily available urotropin is adopted in the invention to lower the cost, thus being easy for industrial large-scale production. Compared with the prior art, the yield of the method involved in the invention is increased to 65% by three-step reaction, also use of expensive di-tert-butyl iminodicarboxylate and cesium carbonate is avoided, the cost is reduced, the operation is simple, and the conditions are mild. Therefore, the method is suitable for industrial preparation of Vilanterol and its key intermediate (5R)-5-(2, 2-dimethyl-4H-1, 3-benzodioxin-6-yl)-1, 3 oxazolidine-2-one. (formula of compound 2).
Owner:SHANGHAI INST OF PHARMA IND +1
4-acetoxypiperidine hydrochlorate preparation method
InactiveCN1763007AThe total yield of the four-step reaction is improvedOrganic chemistryChemical synthesisPyrocarbonic acid
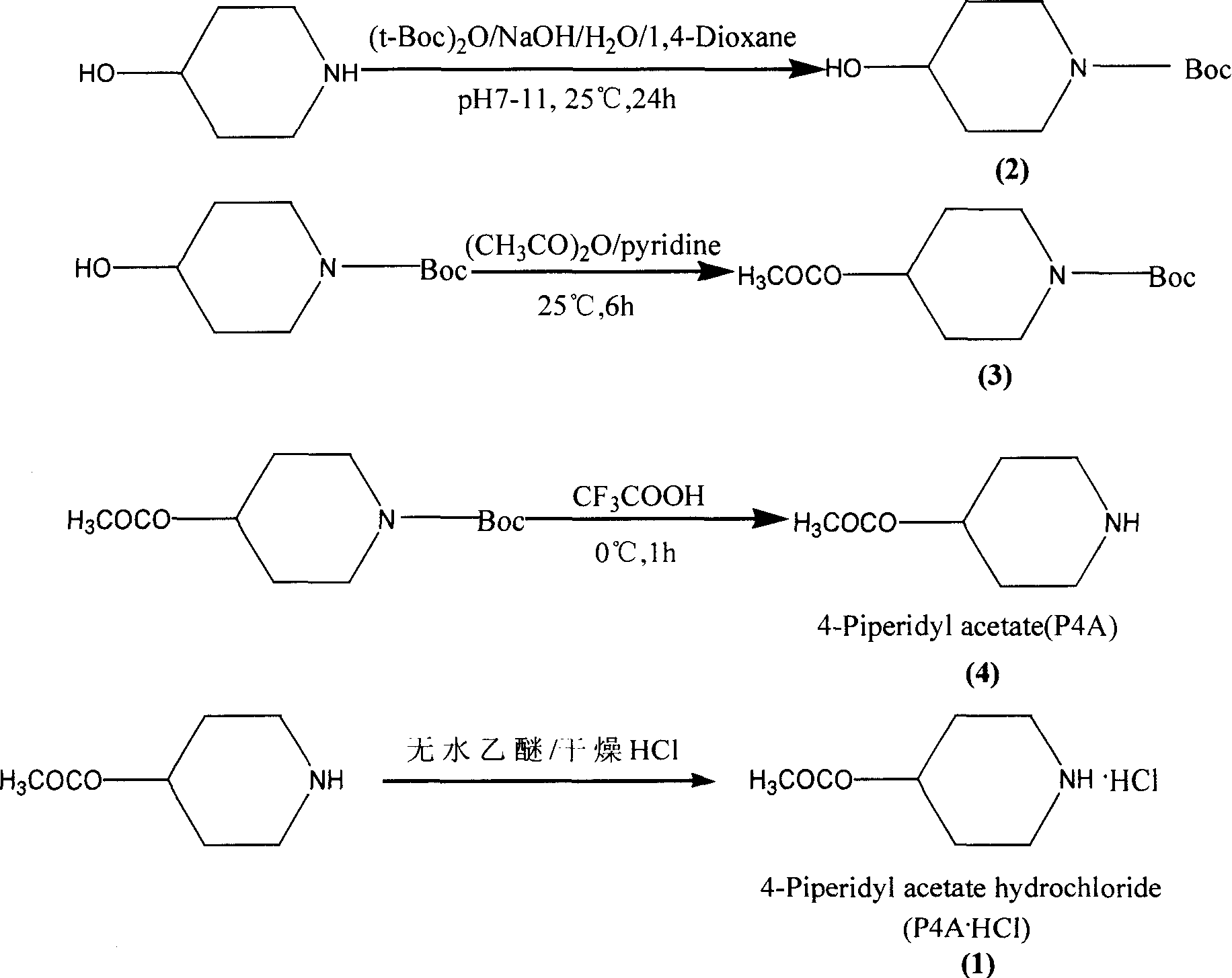
The present invention belongs to the field of chemical synthesis, relates to PET image developer preparing technology, and is especially the synthesis process of 4-acetoxyl piperidine (P4A) hydrochloride. The present invention adopts 4-hydroxyl piperidine hydrochloride as main material, pyrocarbonic acid di-tert-butyl ester in homogeneous alkaline reaction system for amino radical protection and acetic anhydride to acetylate hydroxyl radical before eliminating protecting radical, and introduces dry hydrogen chloride gas into anhydrous ethyl ether to obtain 4-acetoxyl piperidine (P4A) hydrochloride as the destination product. The process has high destination product yield of 71 %, mild reaction condition and simple operation.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Method for synthesizing sugary derivative used for post polymerization modification by double-click chemical combination
ActiveCN110407899AEasy to manufactureSugar derivativesSugar derivatives preparationThiolTert-Butyloxycarbonyl protecting group
The invention relates to a method for synthesizing a sugary derivative used for post polymerization modification by double-click chemical combination. The method comprises the steps: firstly, carryingout a reaction of di-tert-butyl dicarbonate (Diboc) with aminomethyl propanediol (AMPD), protecting an amino group in AMPD with tert-butoxycarbonyl, and then introducing terminal alkyne and terminalolefin at the tail end of the compound by a Williamson etherification reaction; and then carrying out CuAAC reaction on terminal alkyne with acetyl-protected azido sugar (R-N3). Compared with the prior art, the method combines the advantages of CuAAC and Thiol-ene click chemistry, provides a simple and fast way for synthesis of active functional groups which can be subjected to post polymerizationmodification, and has important guiding significance for synthesis of the sugary polymer with controllable molecular weight, narrow molecular weight distribution and biological function.
Owner:SHANGHAI INST OF TECH
Merariveron preparation method
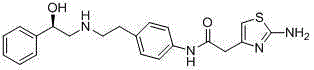
ActiveCN105111165AEasy to operateRaw materials are cheap and easy to getOrganic chemistryBulk chemical productionStyrene oxidePalladium on carbon
The invention discloses a merariveron preparation method which includes the following steps that a, amino protection is carried out, aminobenzyl cyanide and di-tert-butyl dicarbonate react and protect para-position amine to obtain Boc aminobenzyl cyanide; b, nitrile reduction is carried out, hydrogen reduction nitrile is added to the Boc aminobenzyl cyanide under the action of a reduction catalyst of raney nickel or palladium on carbon, and Boc 2-(4-aminophenyl)ethylamine is obtained; c, condensation reaction is carried out, (R)-styrene oxide and the Boc 2-(4-aminophenyl)ethylamine react to obtain (R)-2-((4-Boc amine phenethyl) amine)-1-phenylethanol; d, deprotection is conducted, Boc groups are removed from the (R)-2-((4-Boc amine phenethyl) amine)-1-phenylethanol under the action of trifluoroacetic acid, and (R)-2-((4-amine phenethyl) amine)-1-phenylethanol is obtained; e, ester-amide condensation is carried out, the (R)-2-((4-amine phenethyl) amine)-1-phenylethanol and 2-aminothiazole-4-acetic acid are condensed under the action of a coupling reagent to obtain a target product of merariveron. The method is easy to operate and implement, raw materials are cheap and easy to obtain, reaction efficiency is high, byproducts are few, and repeatability is good.
Owner:保定博洋生物科技有限公司
Room-temperature molten salt and preparation method and application thereof
The invention relates to room-temperature molten salt and a preparation method and application thereof and belongs to the technical field of low-temperature electrolytic aluminum. The room-temperature molten salt is composed of a cation portion and an anion portion. The general formula of the cation portion is [AlCl2.nBase] <+>, wherein Base is one of ethylene carbonate or propylene carbonate or butene carbonate or dimethyl carbonate or diethyl carbonate or methyl ethyl carbonate or diphenyl carbonate or di-tert-butyl dicarbonate, and n ranges from 2 to 20. The anion portion is AlCl4-. According to the room-temperature molten salt and the preparation method and application thereof, a low-temperature co-molten system is found and compounded for the first time; all raw materials of the system are converted into products; purification and separation are not needed; and the room-temperature molten salt and the preparation method and application thereof meet atom economy. In addition, the room-temperature molten salt and the preparation method and application thereof have the beneficial effects of being high in electric conductivity, small in viscosity, stable in air, insensitive to water and low in cost.
Owner:NORTHEASTERN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

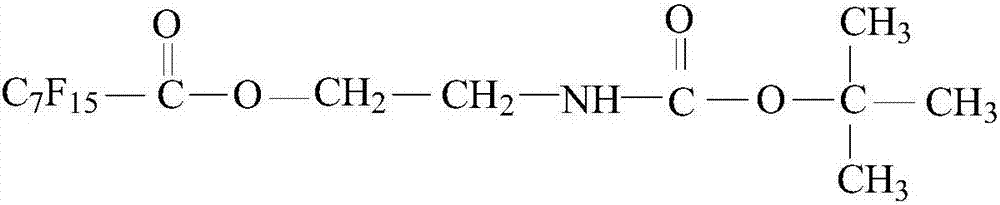
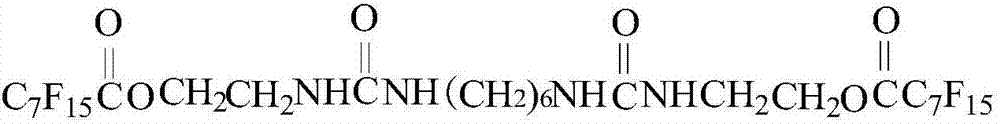









![Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c4e5bc10-490b-4118-8859-0b9646c67500/BSA0000093282790000011.png)
![Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c4e5bc10-490b-4118-8859-0b9646c67500/BSA0000093282790000021.png)
![Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c4e5bc10-490b-4118-8859-0b9646c67500/BSA0000093282790000031.png)