Amide arylpiperazine derivatives, their preparation method, and their application in benign prostatic hyperplasia resistance
The technology of a compound, triethylamine, is applied in the field of preparation of drugs for the treatment of benign prostatic hyperplasia, which can solve problems such as clinical use restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
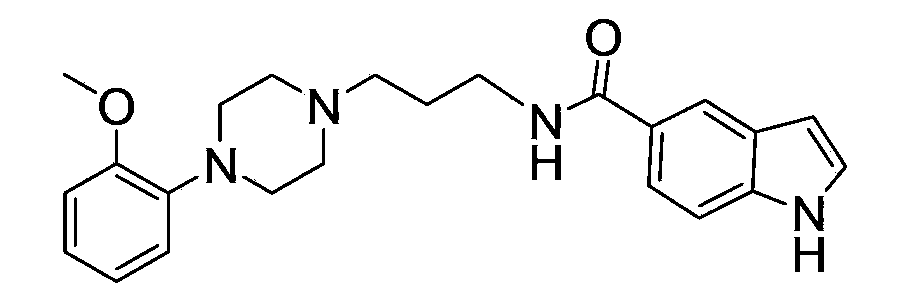
[0097] Embodiment 1: the preparation of compound 1
[0098] Dissolve 3-bromopropylamine hydrobromide (0.01mol) in 10% sodium hydroxide solution (50mL), extract with dichloromethane (3×50mL), dry the organic layer with anhydrous sodium sulfate, filter and concentrate Finally, the obtained free base (1.2 g) and di-tert-butyl dicarbonate (0.011 mol) were mixed and stirred in dichloromethane for 5 hours, the reaction solution was washed with water, dilute hydrochloric acid, and saturated sodium bicarbonate solution successively, and the organic layers were combined. Dry over anhydrous sodium sulfate, filter, and concentrate to obtain a colorless oily liquid (2.2 g, yield 92%).
[0099] React the colorless oily liquid obtained in the previous step with o-methoxyphenylpiperazine (molar ratio 1:2), use dichloromethane as solvent, stir at room temperature for 6-10 hours, add water to the reaction solution, and combine the organic layers , dried, recovered the solvent, and separated t...
Embodiment 2
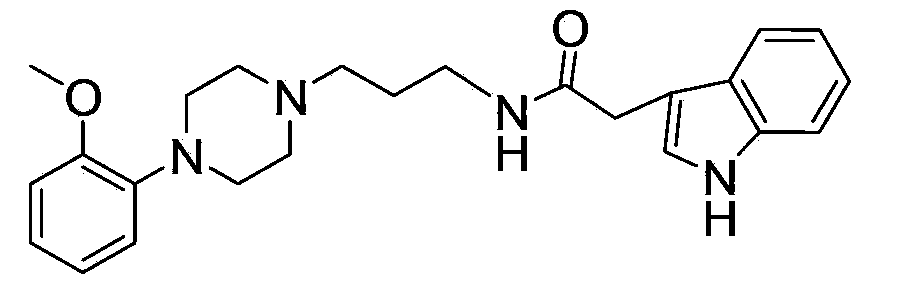
[0102] Embodiment 2: the preparation of compound 2
[0103] The light yellow oil represented by formula (11) was prepared according to the method of Example 1.
[0104] Dissolve the light yellow oil obtained in the previous step (0.5g, 0.0020mol) in dichloromethane (50mL), add DIPEA (0.5mL), add HATU (0.76g) and mix with 3-indoleacetic acid (0.35g) A solution in DMF (20 mL) was protected with nitrogen and stirred at room temperature for 6-10 hours. The reaction solution was washed with water, the organic layers were combined, dried over anhydrous sodium sulfate, the solvent was recovered, and separated by silica gel column chromatography (100-200 mesh silica gel sample was mixed, 300-400 mesh silica gel column was packed), ethyl acetate was used as the eluent, and Thin-layer chromatography detection, collecting the ethyl acetate-triethylamine volume ratio of 40:1 on the thin-layer plate, the fraction with a ratio shift value of 0.4~0.6 was obtained, and 0.63g of a pink viscou...
Embodiment 3
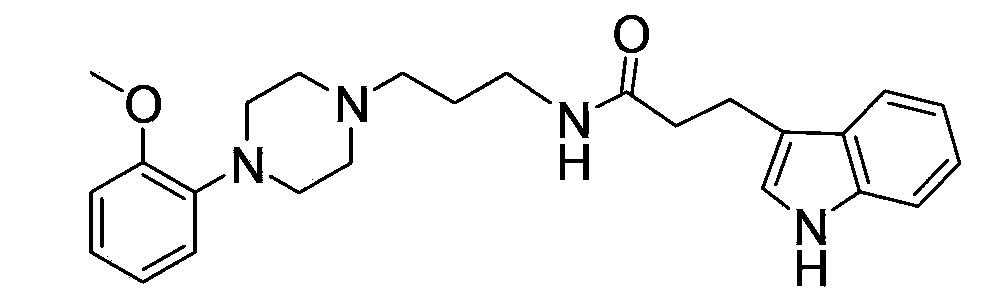
[0105] Embodiment 3: the preparation of compound 3
[0106] The light yellow oil represented by formula (11) was prepared according to the method of Example 1.
[0107] Dissolve the light yellow oil obtained in the previous step (0.5g, 0.0020mol) in dichloromethane (50mL), add DIPEA (0.5mL), add HATU (0.76g) and 3-indolepropionic acid (0.38g) The solution mixed with DMF (20 mL) was protected with nitrogen and stirred at room temperature for 6-10 hours. The reaction solution was washed with water, the organic layers were combined, dried over anhydrous sodium sulfate, the solvent was recovered, and separated by silica gel column chromatography (100-200 mesh silica gel sample was mixed, 300-400 mesh silica gel column was packed), ethyl acetate was used as the eluent, and Thin-layer chromatography was used to collect the fraction with a ratio shift of 0.4-0.6 when the volume ratio of ethyl acetate-triethylamine was 40:1 on the thin-layer plate, and 0.60 g of the product was obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com