Synthesis method for GHK tripeptide
A synthetic method, the technology of -OH, is applied in the field of synthesizing GHK tripeptide to achieve simple operation steps, help separation and purification, and increase lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
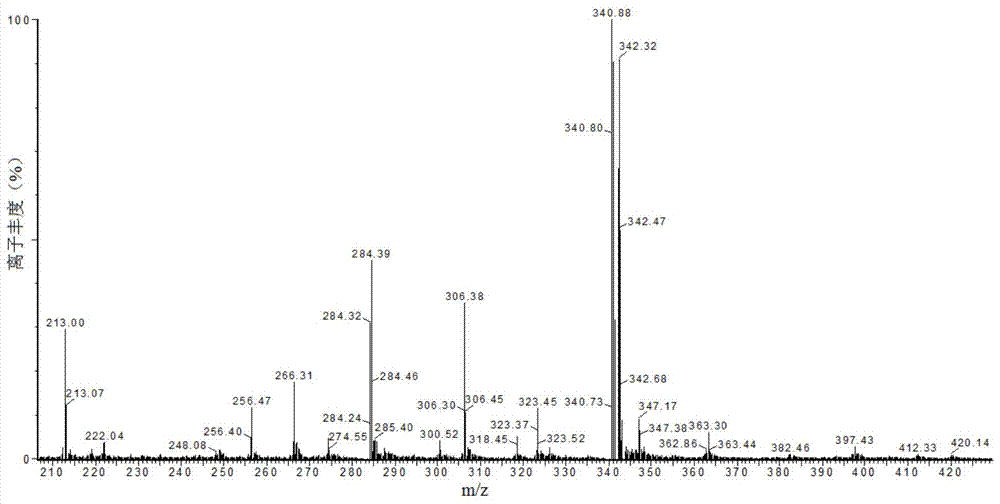
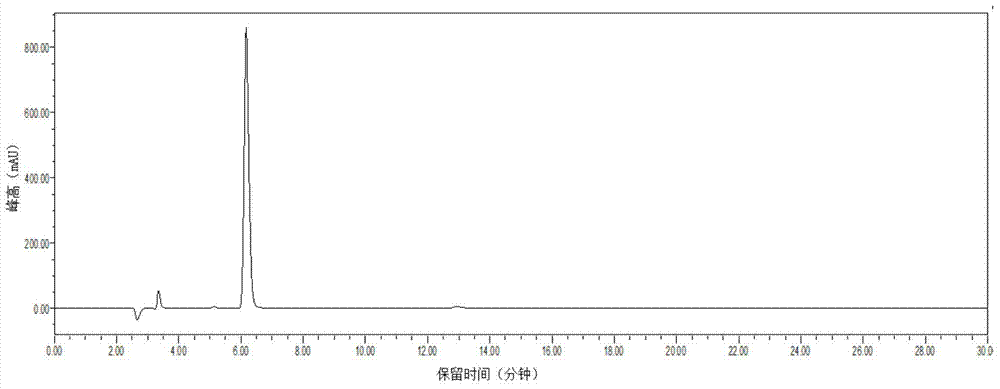
Image
Examples
Embodiment 1
[0025] 1. Synthesis of H-Lys(Ac)-OH
[0026] Add 10g (0.07mol) lysine and 40mL distilled water into the reaction flask, stir at room temperature, adjust the pH value to 10 with 10% sodium hydroxide aqueous solution, and slowly add 15.2g (0.08mol) p-nitrobenzene acetate The mixture of ester (provided by Shanghai Puzhen Biotechnology Co., Ltd.) and 80mL 1,4-dioxane was stirred at room temperature for 4 hours, filtered, the filtrate was concentrated under reduced pressure, extracted with ethyl acetate, and the aqueous phase was used with a mass fraction of 10% citric acid aqueous solution adjusted the pH value to 3, extracted with ethyl acetate, the organic phase was dried with anhydrous sodium sulfate and filtered under reduced pressure, the filtrate was concentrated to dryness under reduced pressure to obtain H-Lys(Ac)-OH, the yield 96%.
[0027] 2. Synthesis of Boc-Gly-COOSu
[0028] Add 10g (0.057mol) Boc-Gly-OH and 100mL 1,4-dioxane into the reaction flask, stir at room te...
Embodiment 2
[0039] In step 1 of the synthesis of H-Lys(Ac)-OH in this example, add 10 g (0.07 mol) of lysine and 40 mL of distilled water into the reaction flask, stir at room temperature, and adjust the pH with 10% sodium hydroxide aqueous solution value to 11, slowly add a mixture of 26.6g (0.0.14mol) p-nitrophenyl acetate (provided by Shanghai Puzhen Biotechnology Co., Ltd.) and 250mL 1,4-dioxane, and stir at room temperature for 5 hours. The other steps of the steps are the same as in Example 1 to obtain H-Lys(Ac)-OH. The other steps were the same as in Example 1 to obtain the GHK tripeptide.
Embodiment 3
[0041] In step 3 of the synthesis of Boc-Gly-His(Boc)-OH in this example, 17.8g (0.0.114mol) of histidine and 9.6g (0.114mol) of sodium bicarbonate were dissolved in 30mL of distilled water, and the resulting solution was added dropwise Into the 1,4-dioxane solution of Boc-Gly-COOSu obtained in step 2, stirred at room temperature for 20 hours, then added dropwise 24.6g (0.114mol) of di-tert-butyl dicarbonate and 200mL of 1,4-dioxane Add 9.3 g (0.228 mol) of sodium hydroxide to the hexacyclic mixture, and react at room temperature for 3 hours. Other steps in this step are the same as in Example 1 to obtain Boc-Gly-His(Boc)-OH. The other steps were the same as in Example 1 to obtain the GHK tripeptide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com