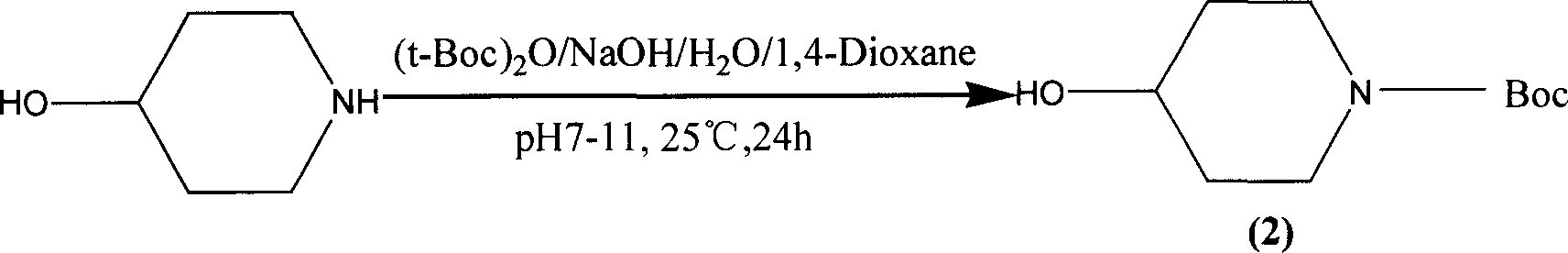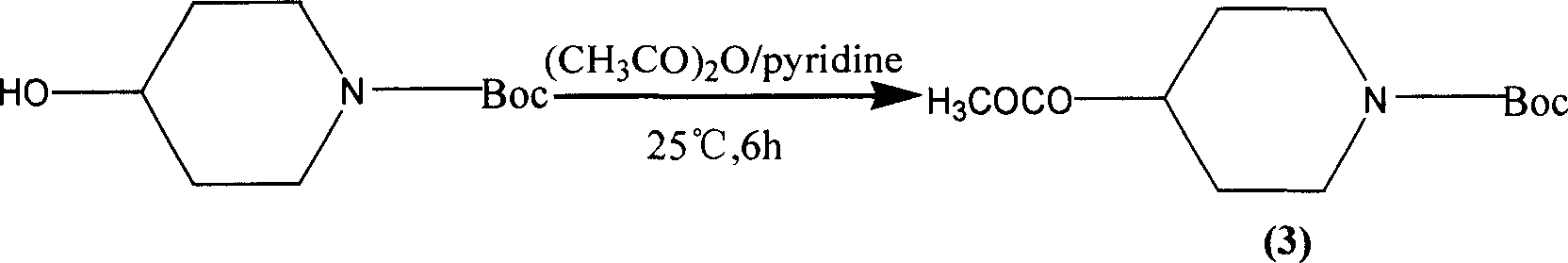4-acetoxypiperidine hydrochlorate preparation method
A technology of acetoxypiperidine and tert-butoxycarbonylpiperidine, which is applied in the field of synthesis of 4-acetoxypiperidine hydrochloride, can solve the problems of heterogeneity and low reaction yield, and achieve high yield Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
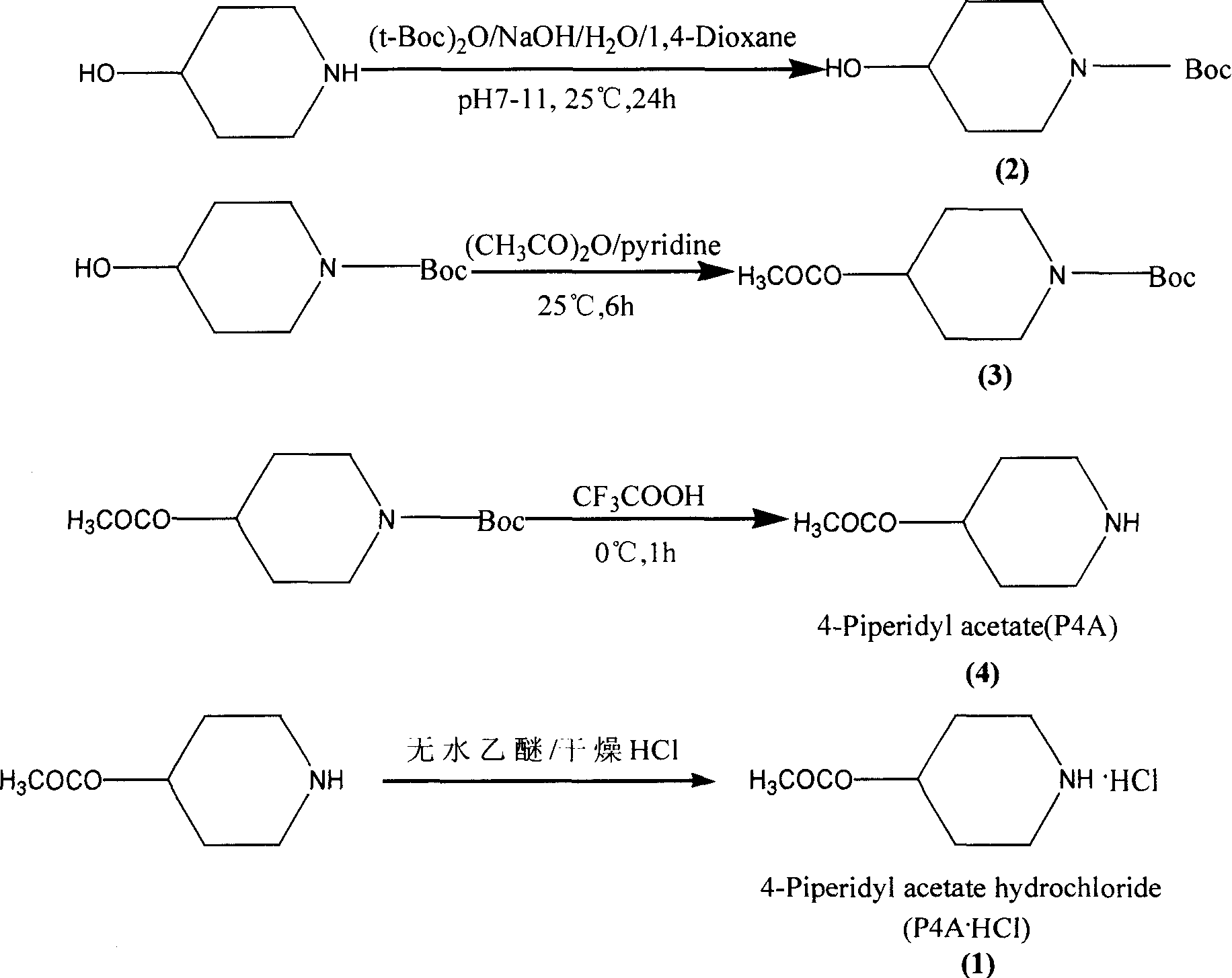
[0035] Preparation of 4-hydroxy-N-tert-butoxycarbonylpiperidine (2) (Ming-Rong Zhang literature method):
[0036] 10.0g (72.73mmol) of 4-hydroxypiperidine hydrochloride was dissolved in 20ml of distilled water, 50ml of 0.5N NaOH solution was added and 18g (82.57mmol) of di-tert-butyl dicarbonate was added in batches, and vigorously stirred at 25°C for 1 hour, The reaction was extracted with chloroform, the chloroform layer was washed with water, 27% ammonia water, and saturated sodium chloride, the organic layer was dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a light yellow oil, which was confirmed to be 4-hydroxypiperidine by structure , the reason for the analysis was that the reaction system was not alkaline enough, so the product required for the experiment did not react.
Embodiment 2
[0038] Preparation of 4-hydroxy-N-tert-butoxycarbonylpiperidine (2):
[0039] 10.0g (72.73mmol) of 4-hydroxypiperidine hydrochloride was dissolved in 80ml of 1N NaOH, under ice cooling, 18g (82.57mmol) of di-tert-butyl dicarbonate was added in batches, and at the same time, 1N NaOH was used to adjust the pH to maintain 8-9 (approximately 100ml 1N NaOH), remove the ice bath after adding, stir overnight at room temperature, extract with chloroform (4×100ml), combine the chloroform layers, wash with water, 7% ammonia water, and saturated sodium chloride three times each, and anhydrous sulfuric acid for the organic layer After drying over sodium, the solvent was distilled off under reduced pressure to obtain a pale yellow oil, which solidified after standing overnight to 11.0 g, with a yield of 75.0%. The product can be directly carried out to the next step without purification.
Embodiment 3
[0041] Preparation of 4-hydroxy-N-tert-butoxycarbonylpiperidine (2):
[0042] 10.0g (72.73mmol) of 4-hydroxypiperidine hydrochloride was dissolved in 80ml of 1N NaOH and 100ml of 1,4-dioxane, under ice cooling, 18g (82.57mmol) of di-tert-butyl dicarbonate was added in batches , while using 1N NaOH to adjust the pH to maintain 7-8 (approximately 100ml 1NNaOH), remove the ice bath after adding, stir at room temperature overnight, then add 100ml of water, evaporate 1,4-dioxane under reduced pressure, and extract with chloroform ( 4 × 100ml), the chloroform layers were combined, washed three times with water, 7% ammonia water, and saturated sodium chloride, the organic layer was dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a light yellow oil, which solidified after standing overnight. 14.0g, The yield was 94.0%, and the product could be directly carried out to the next reaction without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com