Method for preparing primary amine carbon nano tube
A technology of carbon nanotubes and nanotubes, which is applied in the field of chemically modifying the surface of carbon nanotubes and carbon nanotubes. It can solve the problems of complex steps, harsh reaction conditions, and inability to guarantee amino groups, etc., and achieves simple steps, high reactivity, and extensive use. The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
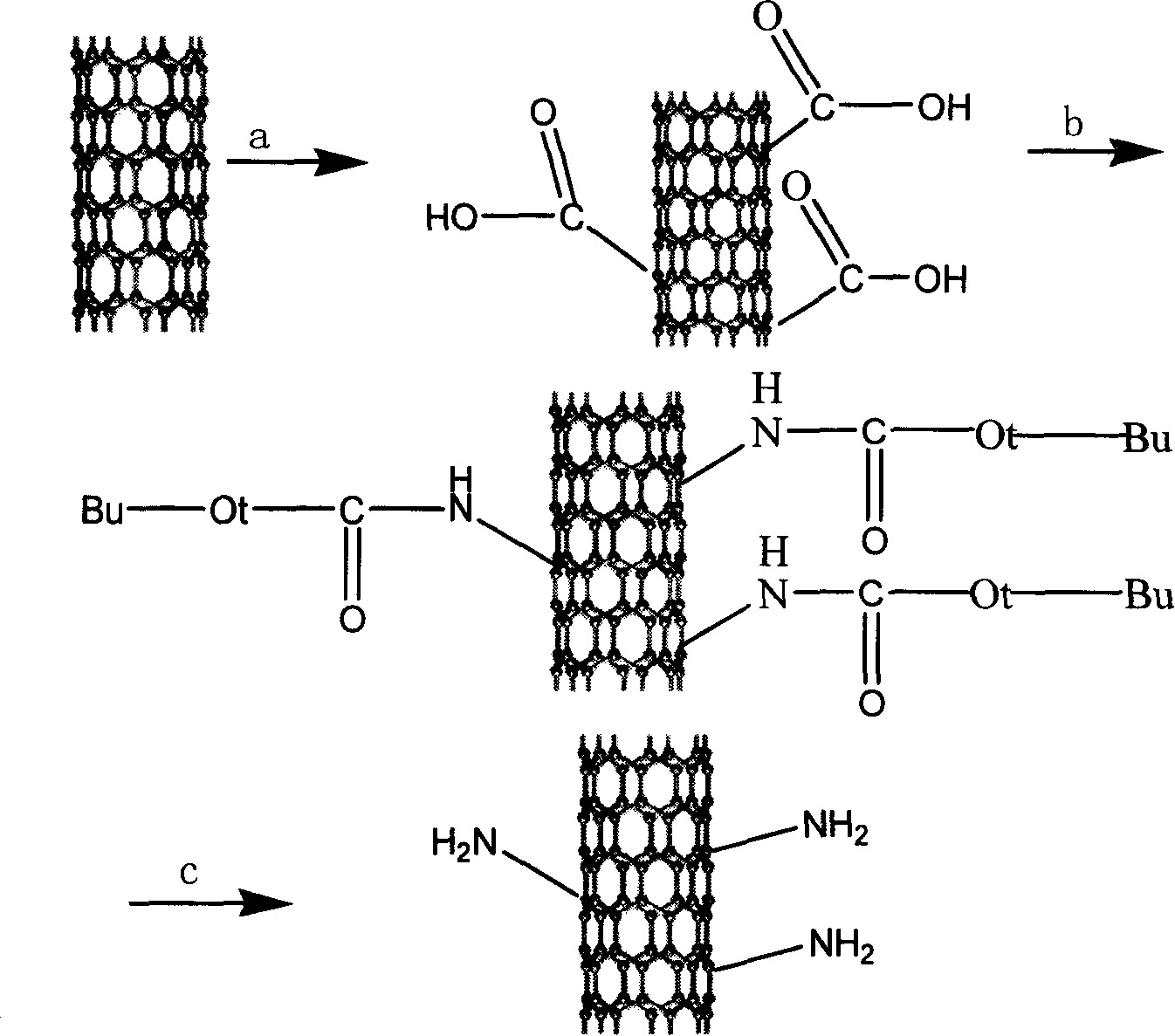
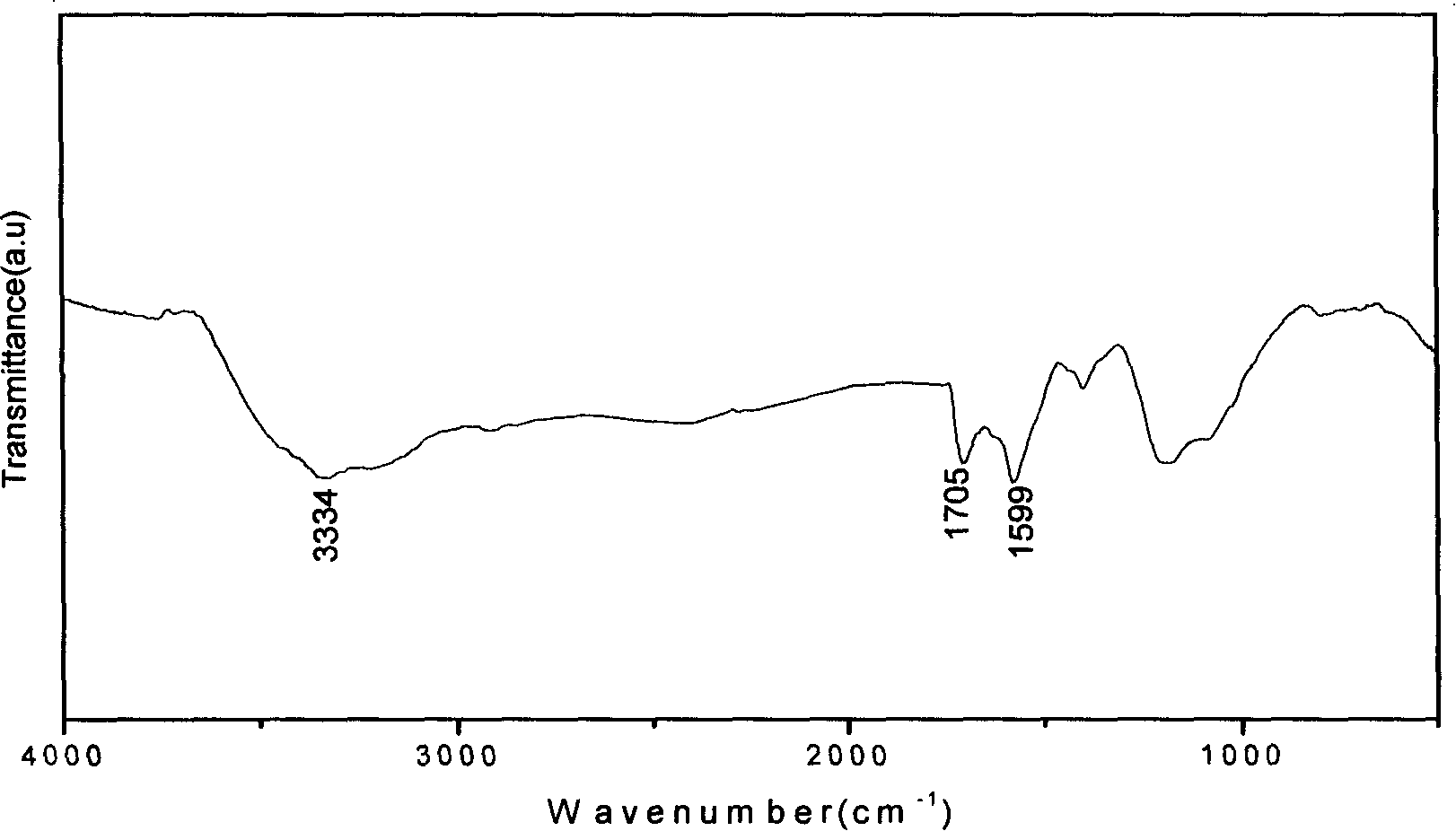
[0020] as attached figure 1 Shown: the first step: mix 500mg SWNT (diameter 1~2nm, purity greater than 90%) with 150ml H 2 SO 4 with HNO 3 mixed solution (98%H 2 SO 4 : 68% HNO 3 = 3:1 vol / vol), and reflux reaction for 4 hours at 35-40° C. and 50 kHz ultrasonic oscillation. Then filter through a vinylidene fluoride membrane with a pore size of 0.45 μm, and wash with water until the pH is neutral. The product was placed in a vacuum oven and dried under vacuum at 40°C for 24h. The above carboxylated and washed product was placed in H 2 SO 4 with H 2 o 2 mixed solution (98%H 2 SO 4 : 30%H 2 o 2 =4:1vol / vol) at 70°C for 2h. Then filter through a vinylidene fluoride membrane with a pore size of 0.45 μm, and wash with water until the pH is neutral. The product was placed in a vacuum oven and dried under vacuum at 40°C for 24h. The infrared spectrum of the product is attached figure 2 shown.
[0021] The second step: take 85 mg of the above-mentioned carboxylated ...
Embodiment 2
[0024] See Example 1 for the first step, and the second step: take 85 mg of the above-mentioned acidified carbon nanotubes and place them in a reaction flask, add 120 mg of di-tert-butyl dicarbonate, 114 mg of sodium azide, and 24.15 mg of tetrabutylammonium bromide, Zinc bromide 7.425mg, tetrahydrofuran 100ml, stirred and refluxed at 40°C for 24h. Filtered and washed several times with THF. Carbon nanotubes bearing tert-butyl carbamate were obtained.
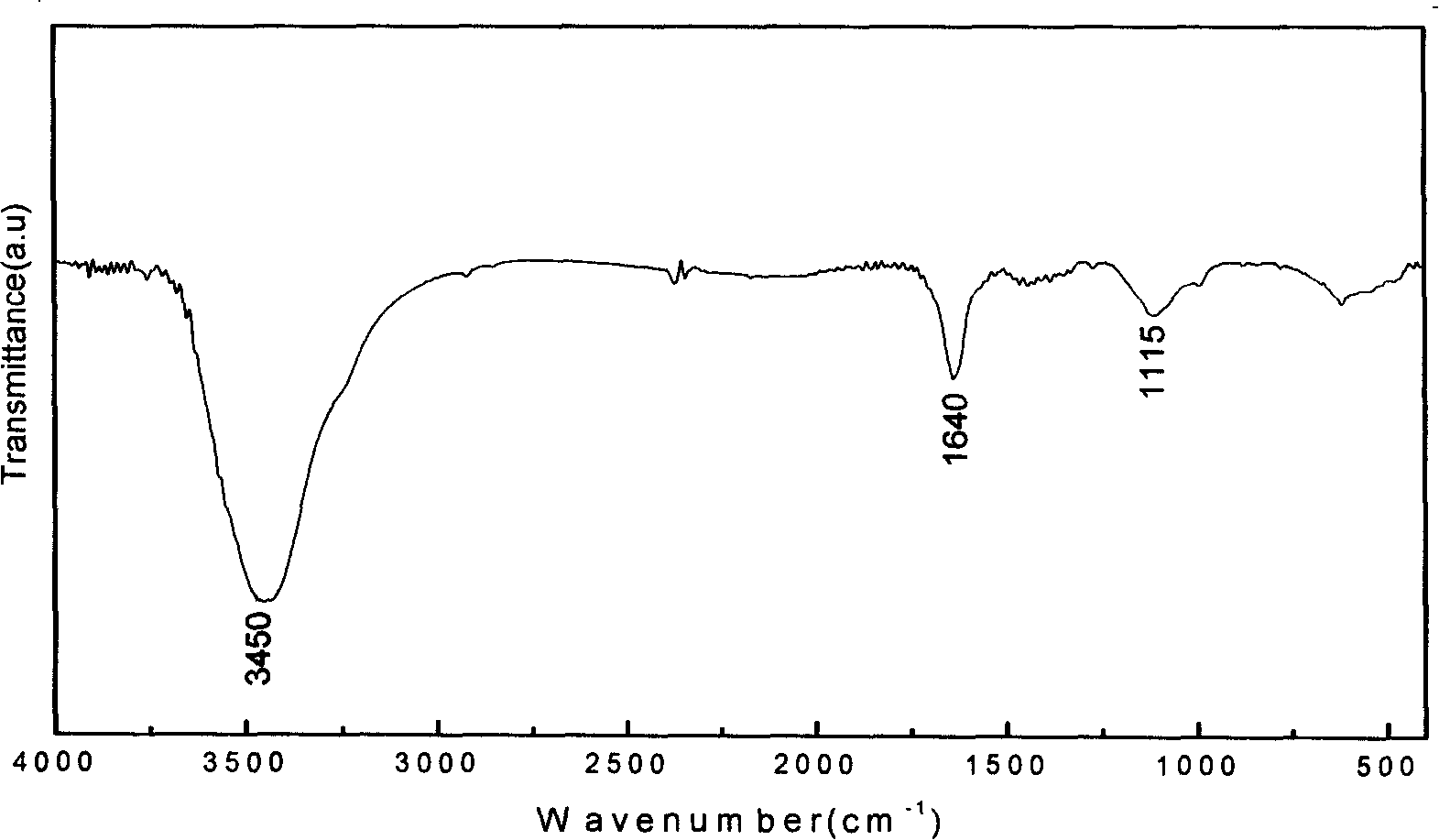
[0025] Step 3: Add 50 mg of carbon nanotubes with tert-butyl carbamate into a mixed solution of 10 ml of hydrochloric acid and 20 ml of ethyl acetate, stir, and react at room temperature for 2 hours to stop the reaction. Filter through a vinylidene fluoride membrane, wash with water, and filter through a fiber membrane with a pore size of 0.45 μm to obtain a black powder.
Embodiment 3
[0027] See Example 1 for the first step, and the second step: take 85 mg of the above-mentioned acidified carbon nanotubes and place them in a reaction flask, add 120 mg of di-tert-butyl dicarbonate, 114 mg of sodium azide, and 24.15 mg of tetrabutylammonium bromide, Zinc trifluoromethanesulfonate 12mg, tetrahydrofuran 100ml, stirred and refluxed at 40°C for 24h. Filtered and washed several times with THF. Carbon nanotubes bearing tert-butyl carbamate were obtained.
[0028]Step 3: Add 50 mg of carbon nanotubes with tert-butyl carbamate into a mixed solution of 10 ml of hydrochloric acid and 20 ml of ethyl acetate, stir, and react at room temperature for 2 hours to stop the reaction. Filter through a vinylidene fluoride membrane, wash with water, and filter through a fiber membrane with a pore size of 0.45 μm to obtain a black powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com