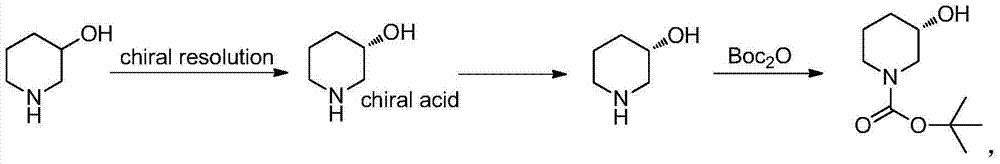
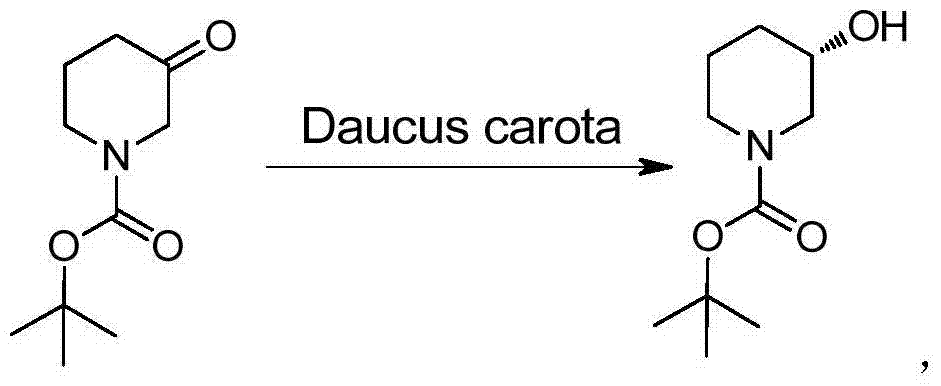
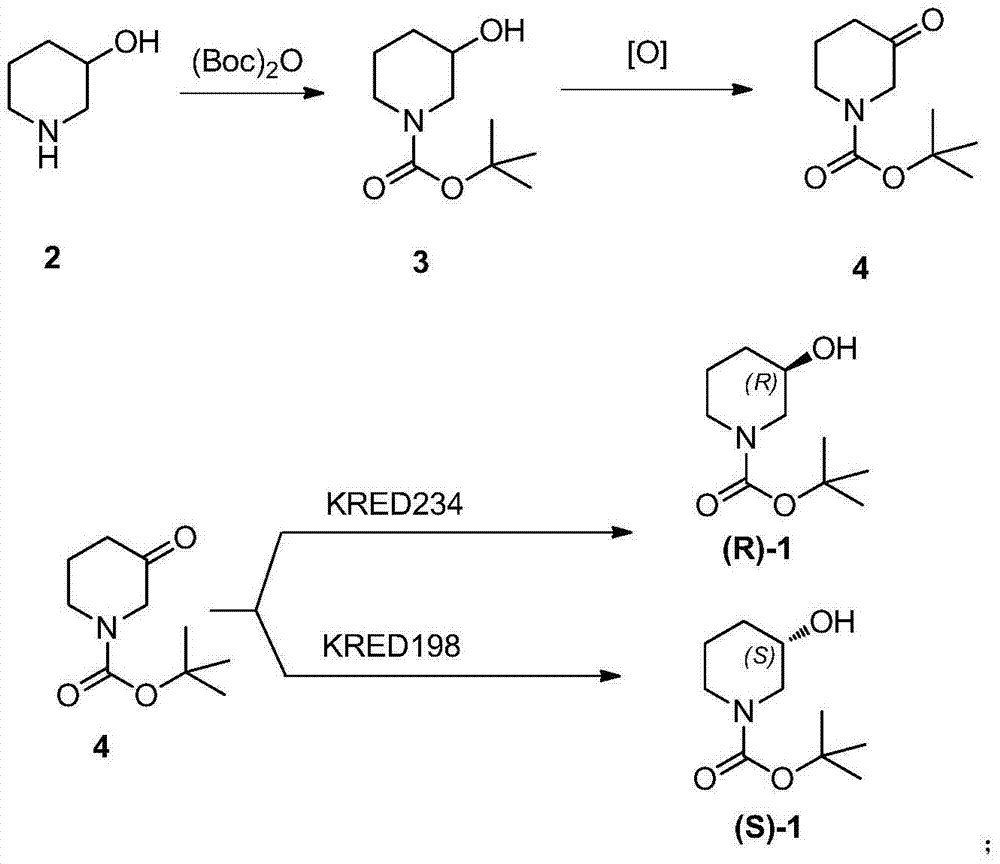
Method for preparing chiral N-tert-butyloxycarboryl-3-hydroxypiperidine
A technology of tert-butoxycarbonyl and hydroxypiperidine, which is applied in the field of preparation of chiral N-tert-butoxycarbonyl-3-hydroxypiperidine, which can solve problems that are difficult to apply to industrial production, difficult to obtain a large amount of catalysts, and insufficient optical purity of products Advanced problems, to achieve the effect of large industrial application value, cheap raw materials, simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Synthesis of N-tert-butoxycarbonyl-3-hydroxypiperidine
[0030] At room temperature, add 100mL of 3-hydroxypiperidine into the reaction flask, add 500ml of water, then add 200g of di-tert-butyl dicarbonate, stir for 12h, add dichloromethane for extraction, and the obtained dichloromethane solution is directly used in the next reaction .
[0031] Two, the synthesis of N-tert-butoxycarbonyl-3-piperidone
[0032] At room temperature, to the dichloromethane solution of the above-mentioned N-tert-butoxycarbonyl-3-hydroxypiperidine, add 500 ml of water and 5 g of 2,2,6,6-tetramethylpiperidine-N-oxide, Adjust the pH value to 9.0 with sodium hydroxide solution, add 100 ml of saturated sodium hypochlorite solution dropwise under stirring, stir for 12 h, separate the dichloromethane layer, and concentrate to obtain a residue that is directly used in the next reaction.
[0033] 3. Synthesis of (R)-N-tert-butoxycarbonyl-3-hydroxypiperidine
[0034] At room temperature, add 50...
Embodiment 2
[0036] 1. Synthesis of N-tert-butoxycarbonyl-3-hydroxypiperidine
[0037] As described in Example 1.
[0038] Two, the synthesis of N-tert-butoxycarbonyl-3-piperidone
[0039] As described in Example 1.
[0040] 3. Synthesis of (S)-N-tert-butoxycarbonyl-3-hydroxypiperidine
[0041] At room temperature, add 500 ml of water, 10 g of ketoreductase KRED198 enzyme powder (Shangke Biomedicine (Shanghai) Co., Ltd.), 90 g of glucose, 0.05 g of NADP, and glucose dehydrogenation GDH105 enzyme powder to the residue obtained above at room temperature (Shangke Biopharmaceutical (Shanghai) Co., Ltd.) 10 g, control the pH value to 7.0, stir for 24 hours, filter, add ethyl acetate to extract three times, combine the organic layer, dry, filter, and concentrate to obtain the crude product; Crystallization in medium yielded 143 g of (S)-N-tert-butoxycarbonyl-3-hydroxypiperidine with a molar yield of 72% and an optical purity of 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com