Tyrosine phthalocyanines derivates, preparation thereof and applications in preparation of photodynamic drugs
A technology of tyrosine phthalocyanines and derivatives, which is applied in the field of photosensitizers, can solve the problems of limiting the expansion of phthalocyanine derivatives, failing to solve the purpose of accumulation, and limiting applications, and achieves excellent photosensitivity and good targeting. Function, effect of good physiological compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the specific steps of tyrosine zinc phthalocyanine synthesis are as follows:
[0038](1) Under the protection of nitrogen, add 8.67mmoL 4-nitrophthalonitrile, 5.79mmoL-Boc-tyrosine ethyl ester, 11.55mmoL potassium carbonate and 20mL N,N-dimethylformamide in sequence, and wait until all After dissolving, it was reacted at 40°C for 8 hours, then washed with dichloromethane and saturated brine respectively, dried over anhydrous sodium sulfate, and finally separated by column chromatography (ethyl acetate:petroleum ether=1:5), and the solution was spin-dried to obtain white The crystalline solid (II) was 2.4626 g, and the yield was 97.7%.
[0039] Compound Identification:
[0040] NMR (δ 1 H, CDCl 3 ):7.72(d,1H);7.26(dd,2H);7.24(dd,2H);7.0(d,2H);3.0(dd,2H);4.5(dd,1H);5.1(d,1H ); 4.2 (dd, 2H); 1.42 (s, 9H); 1.27 (t, 3H).
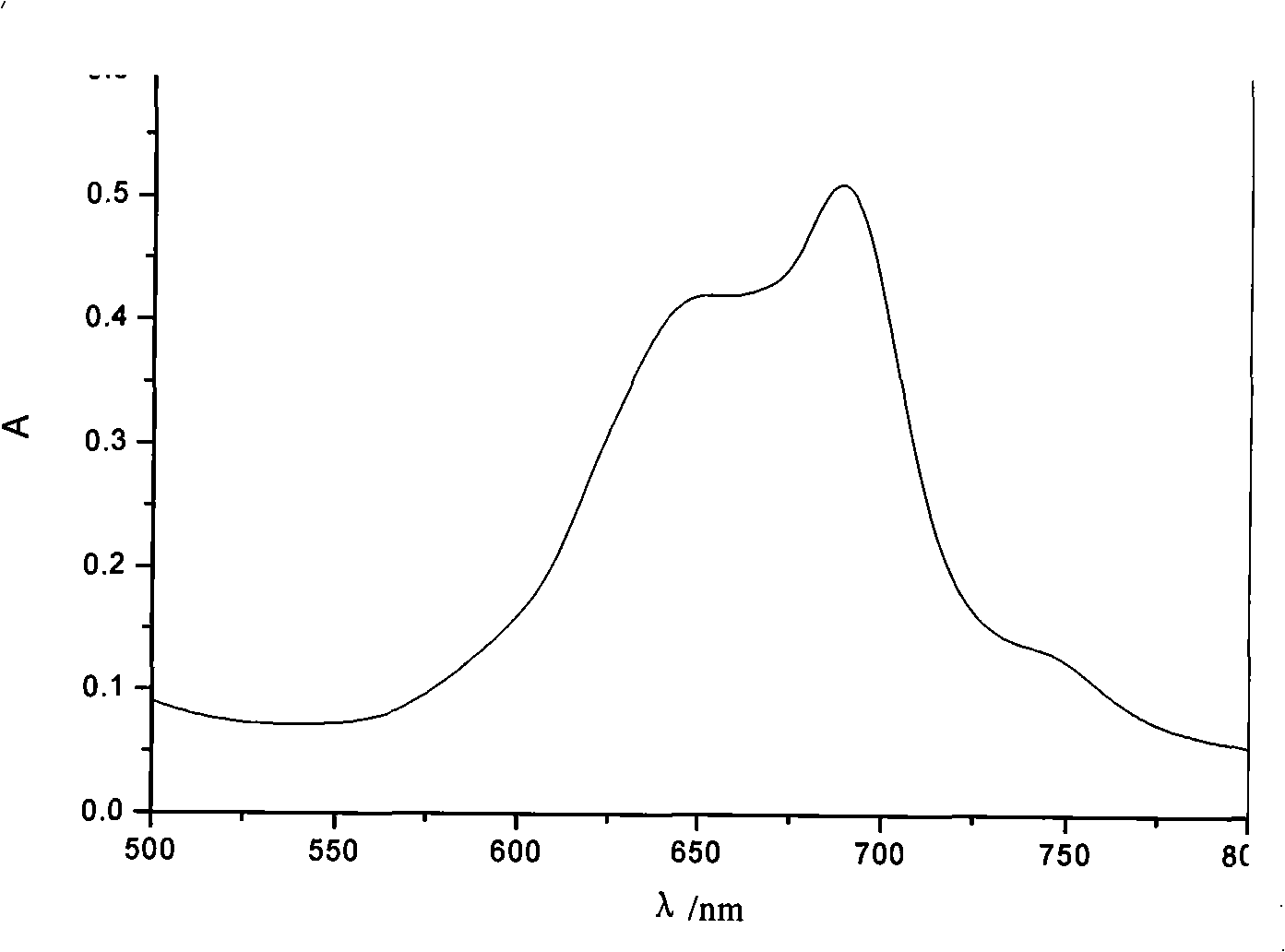
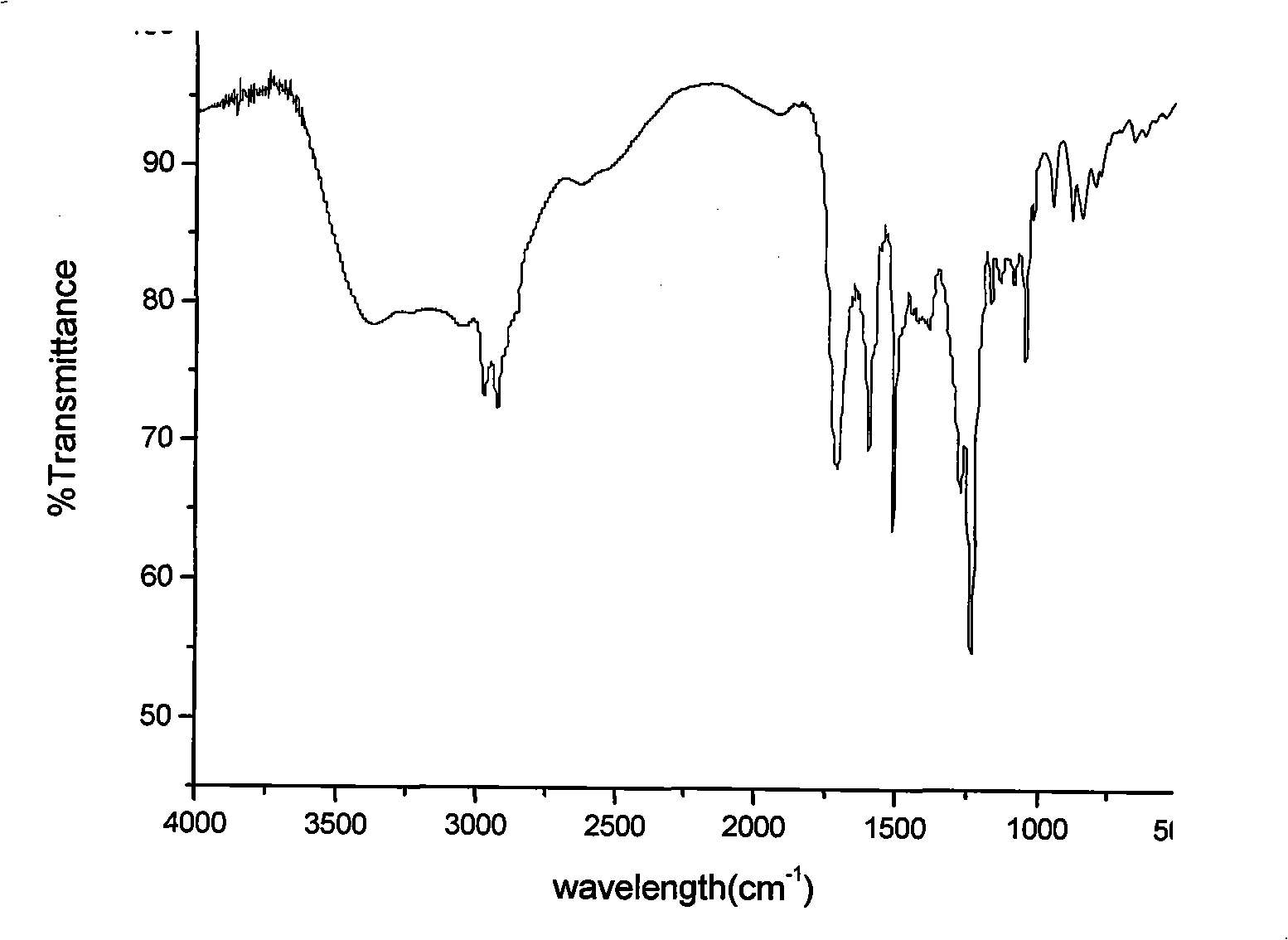
[0041] Infrared absorption (KBr, cm -1 ): 2231 (CN); 1206 (=CH-O-CH=); 3346 (O=C-NH); 1726 (O=C-O).
[0042] Mass spectrometry: m / z 4...
Embodiment 2
[0060] Embodiment 2 is substantially the same as the steps (1), (2), and (3) of Example 1, but without the step (4) of Example 1, the final product is ethyl tyrosine zinc phthalocyanine.
Embodiment 3
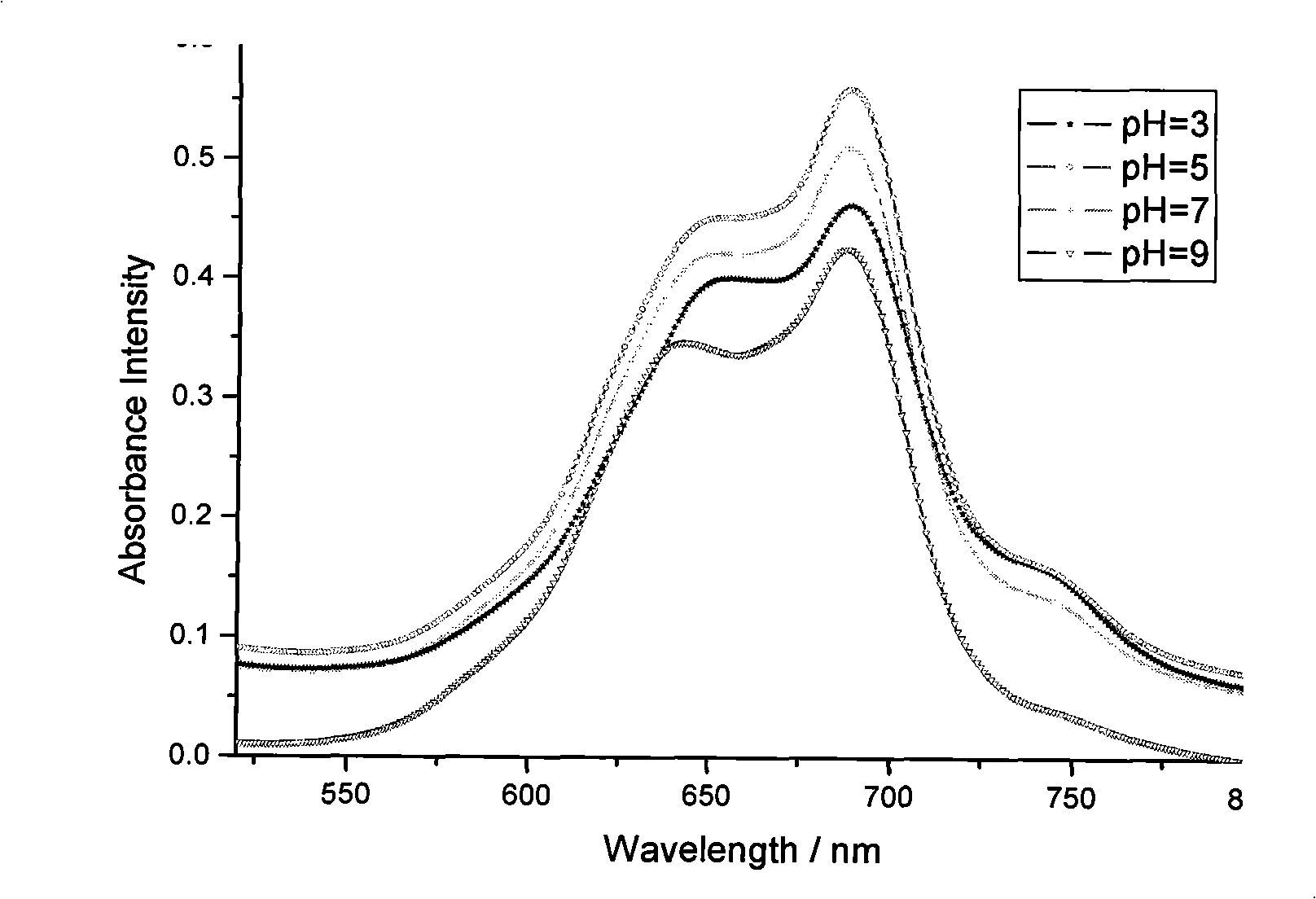
[0061] Example 3 is substantially the same as Example 1, but the pH value of the solution is not adjusted in the step (4) of Example 1, and the final product is sodium tyrosine zinc phthalocyanine. (Since the solution is an alkaline solution at this time, it is impossible for tyrosine to exist, it can only exist in the form of sodium tyrosine)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com