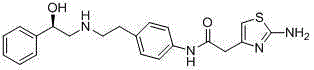
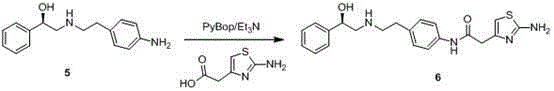
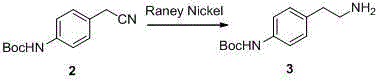Merariveron preparation method
A technology of amino and phenylethyl alcohol, applied in organic chemistry, bulk chemical production, etc., can solve the problems of high yield and removal of chiral hydroxyl groups, and achieve high reaction efficiency, less by-products, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] In a 500mL reaction flask, dissolve 20g of p-aminobenzeneacetonitrile (compound 1) (0.15mol) in 200mL of methanol, and slowly add 50mL of triethanolamine and 35g (0.15mol) of di-tert-butyl dicarbonate under nitrogen protection. After the addition, reflux the reaction at 70°C for 12h. TLC monitors the complete reaction of the raw materials. Adjust the pH of the reaction solution to neutral with dilute hydrochloric acid. Rotary evaporation to remove the solvent methanol, add a certain amount of dichloromethane, and wash the reaction solution with water Three times, the organic phase was separated, and the organic phase was evaporated to obtain 30 g of p-Boc aminobenzene acetonitrile (compound 2).
Embodiment 2
[0034]
[0035] In a 500mL autoclave, add 20g of p-Boc aminobenzene acetonitrile (compound 2) (0.086mol) and 2g of catalyst Raney nickel into 200mL of methanol, and pass hydrogen into the autoclave, the pressure reaches 0.5MPa, the reaction temperature The temperature was 40°C, after 12 hours of reaction, the reaction of the raw materials was monitored by TLC to complete the reaction, the reaction liquid was filtered, and the filtrate was concentrated to obtain 17 g of pure p-Boc aminophenylethylamine (compound 3).
Embodiment 3
[0037]
[0038] In a 500mL autoclave, add 20g of p-Boc aminobenzene acetonitrile (compound 2) (0.086mol) and 1g of catalyst Raney nickel to 200mL of methanol, and feed hydrogen into the autoclave, the pressure reaches 0.5MPa, the reaction temperature The temperature was 40°C, after 20 hours of reaction, the reaction of the raw materials was monitored by TLC to complete the reaction. The reaction solution was filtered and the filtrate was concentrated to obtain 15 g of pure p-Boc aminophenylethylamine (compound 3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com