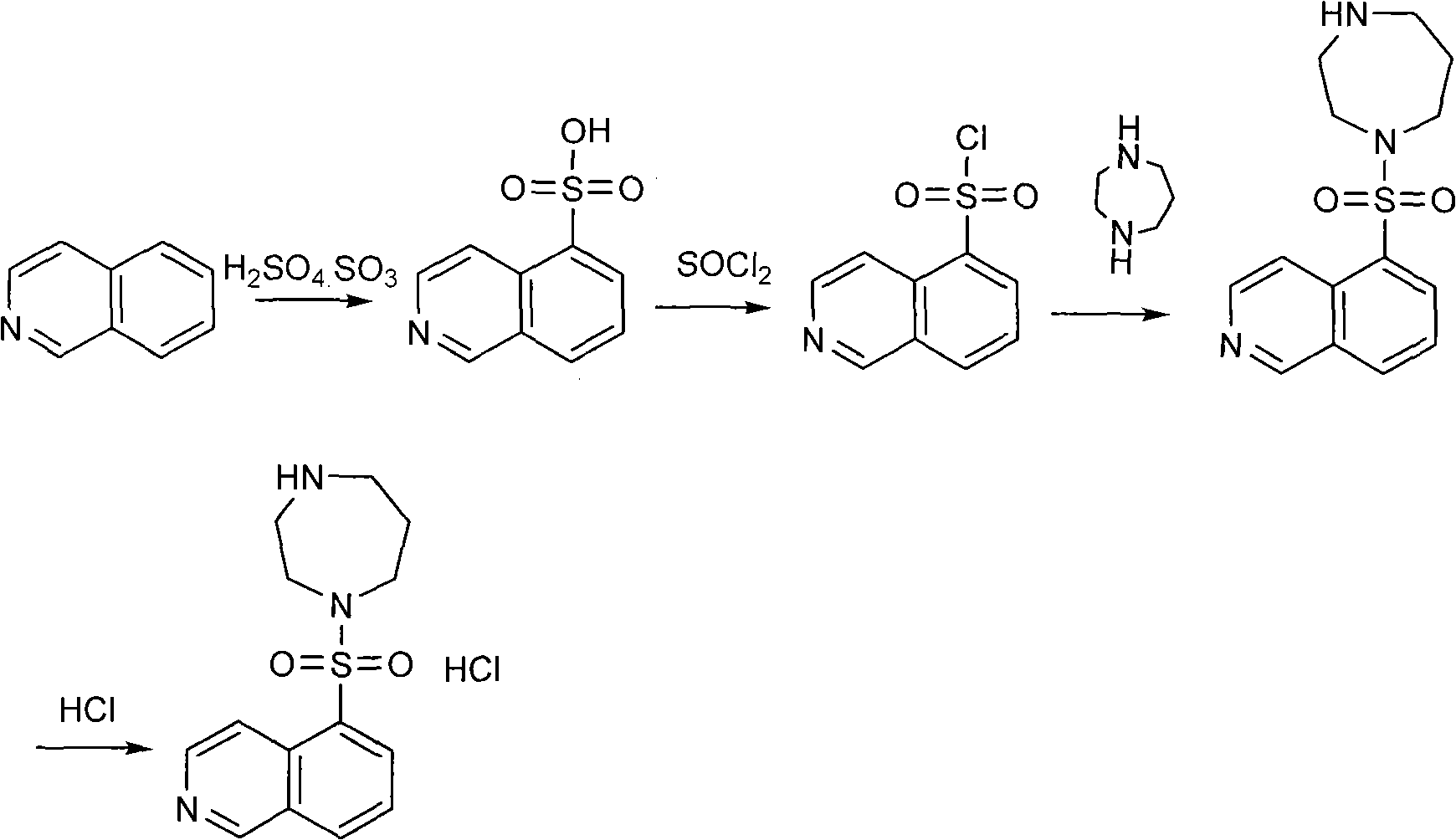
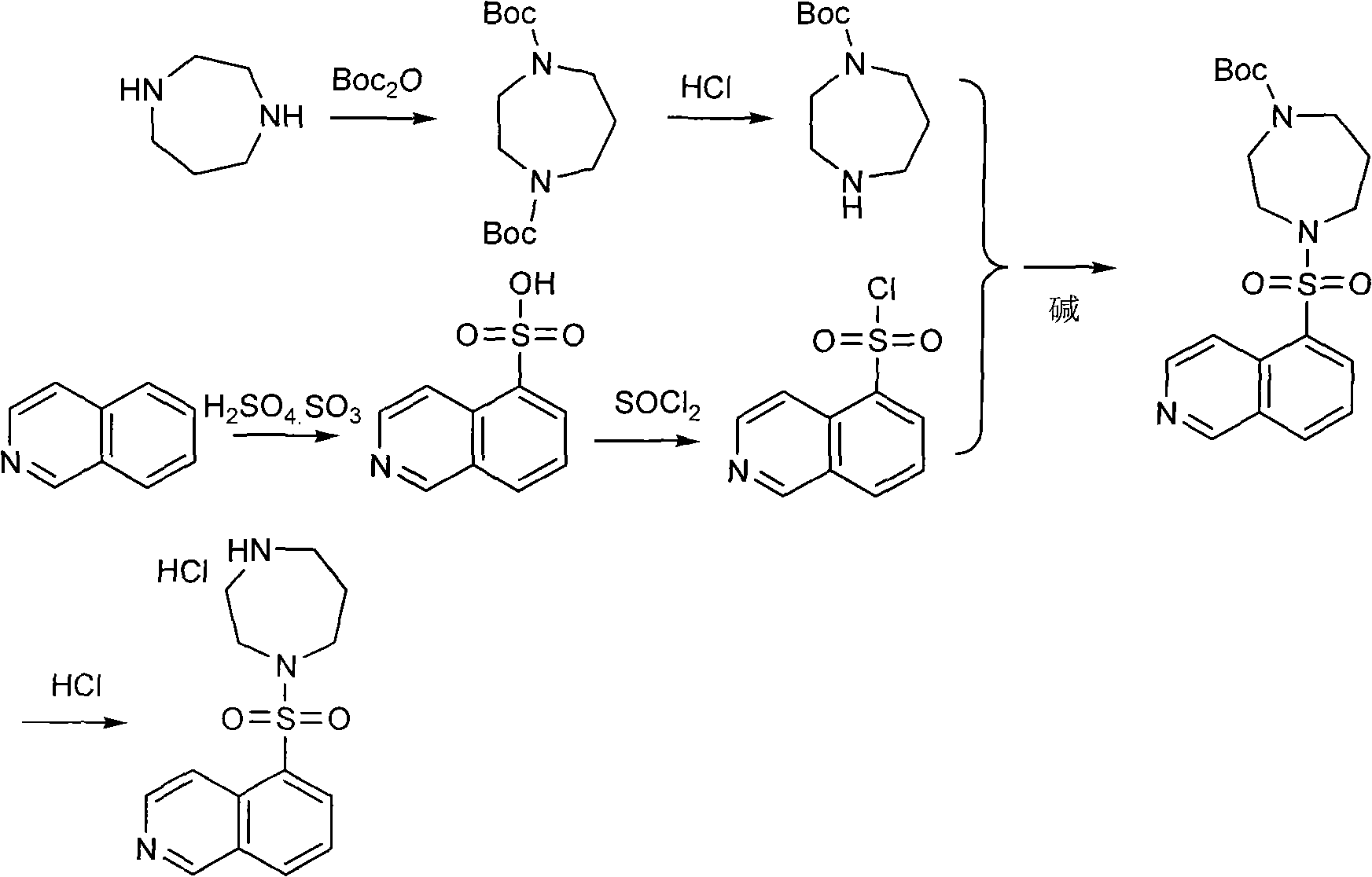
Production method of fasudil hydrochloride
A technology of fasudil hydrochloride and its production method, which is applied in the direction of organic chemistry, can solve the problems of incomplete utilization, easy formation of impurities, high cost, etc., and achieve the effects of full utilization, increased reaction yield, and simple and convenient treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
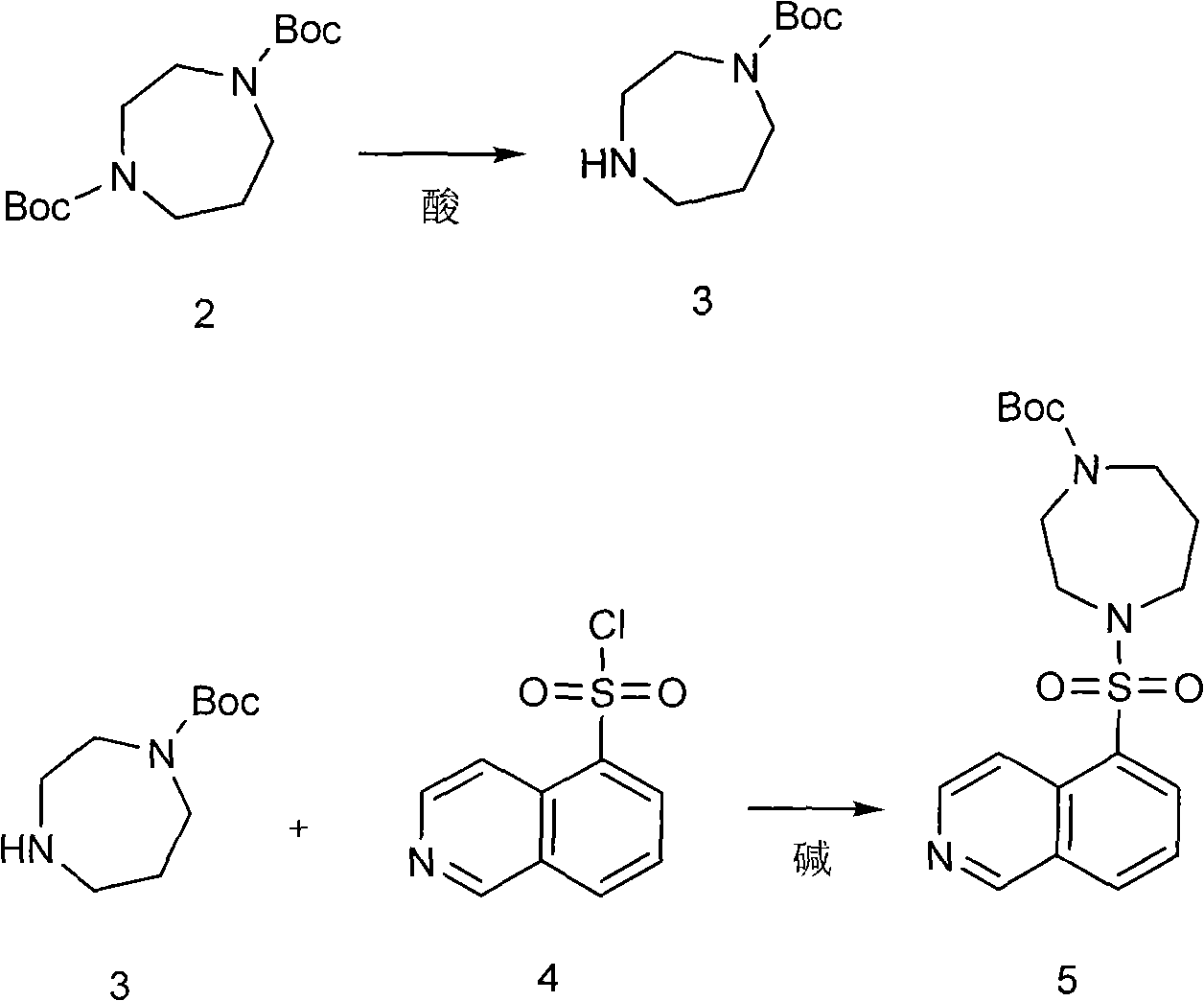
[0026] Example 1 The preparation of 1-tert-butylcarbonate group-1,4-diazepine (compound 3)
[0027] (1) In a single-necked bottle equipped with magnetic stirring, add ethanol and ethyl acetate with a volume ratio of 1:1 mixed solvent (100ml), and slowly add thionyl chloride (17g, 0.143mol) dropwise under cooling in a water bath to obtain An acidic alcoholic solution.
[0028] (2) In a three-necked flask equipped with mechanical stirring, add 1,4-tert-butyl dicarbonate-1,4-diazepine (compound 2) (41.5g, 0.138mol), then add ethanol and ethyl acetate A mixed solvent (200ml) with an ester volume ratio of 1:1; slowly add the acidic solution obtained in step (1) dropwise at room temperature, stir for 2 hours, and filter to obtain 1-tert-butylcarbonate-1,4-di The yield of azepine (compound 3) (15 g, 0.064 mol) was 46.4%. The filtrate was concentrated to obtain 20.6 g of unreacted 1,4-tert-butyldicarbonate-1,4-diazepine (compound 2), with a recovery rate of 49.5%.
example 2
[0029] Example 2 Preparation of 1-(5-isoquinolinesulfonyl)-4-tert-butylcarbonate-1,4-diazepine (compound 5)
[0030] In the there-necked flask equipped with mechanical stirring, add sodium hydroxide (2g, 0.0500mol), water (60ml) and ethanol (60ml), add 1-tert-butylcarbonate group-1,4-diazepine ( Compound 3) (10 g, 0.0427 mol), isoquinoline-5-sulfonyl chloride (compound 4) (9.7 g, 0.0426 mol). Stir at room temperature for 2 hours, concentrate to remove ethanol, extract with ethyl acetate, and concentrate the extract to obtain 1-(5-isoquinolinesulfonyl)-4-tert-butylcarbonate-1,4-diazepine (compound 5), as light yellow sticky substance.
example 3
[0031] Example 3 Preparation of hexahydro-1-(5-isoquinolinesulfonyl)-1H-1,4-diazepine hydrochloride (fasudil hydrochloride)
[0032] The viscous material obtained in Example 2 was dissolved in ethanol, thionyl chloride was added dropwise, filtered, and dried. Hexahydro-1-(5-isoquinolinesulfonyl)-1H-1,4-diazepine hydrochloride (fasudil hydrochloride) (13 g, 0.0397 mol) was obtained with a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com