Method for synthesizing sugary derivative used for post polymerization modification by double-click chemical combination
A click chemistry and post-polymerization technology, which is applied in the direction of sugar derivatives, sugar derivatives, sugar derivatives preparation, etc., can solve the problems of side chain functional group polymerization introduction and polymerization reaction out of control, etc., to achieve molecular weight controllable, narrow The effect of molecular weight distribution and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] 1. Synthesis of Compound 1
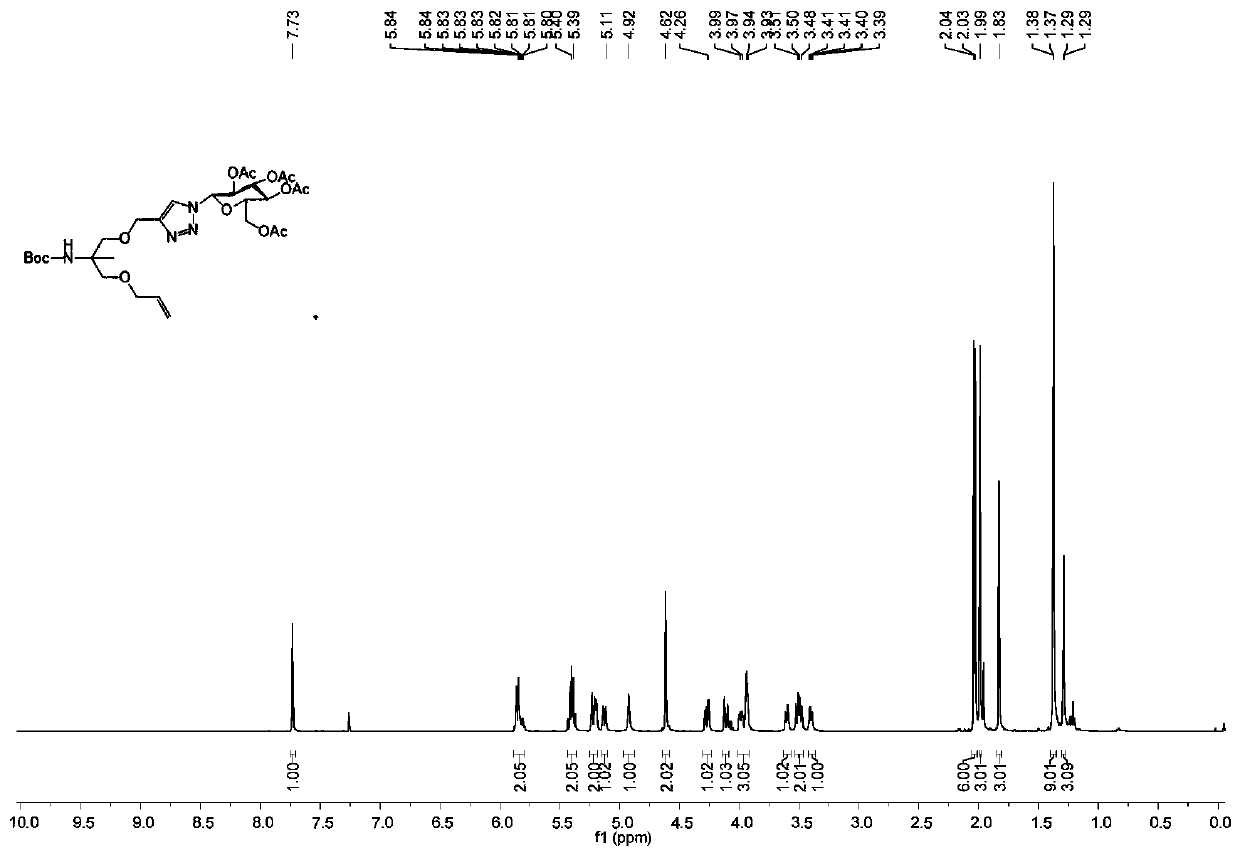
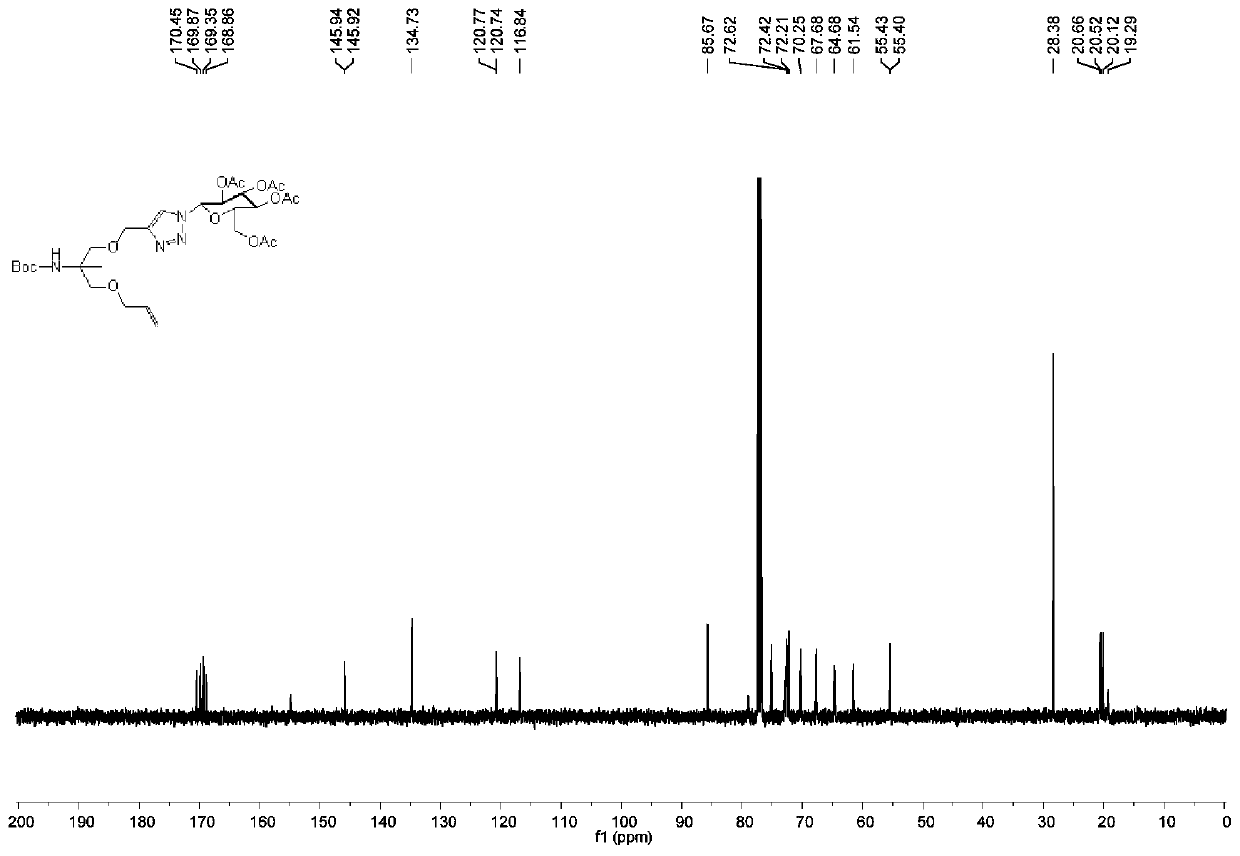
[0070] Weigh aminomethylpropanediol (AMPD) (10.00g, 95.11mmol) and dissolve in 125mL (MeOH:THF=4:1) solvent, add di-tert-butyl dicarbonate (Diboc ) (31.14g, 142.67mmol), sodium bicarbonate (15.98g, 190.22mmol) was added under stirring, stirred evenly, and then reacted at room temperature for 12h, followed by TLC detection. After the reaction was completed, it was washed with ethyl acetate / water, the organic phase was dried with anhydrous sodium sulfate, and then silica gel powder was added to directly spin dry the sample. Silica gel column chromatography (developing agent: PE / EA (v / v)=1:1, Eluent: PE / EA (v / v)=2:1) 19.50 g of white powdery solid (compound 1) was isolated with a yield of 92%. 1 H NMR (500MHz, CDCl 3 )δ=5.10(s,1H),3.71(dd,J=11.1,3.7Hz,2H),3.59(dd,J=11.2,6.6Hz,2H),1.41(s,9H),1.15(s,3H ).
[0071] 2. Synthesis of Compound 2
[0072] Weigh compound 1 (10.00g, 48.70mmol) and dissolve it in 50mL N,N-dimethylformamide, put it ...
Embodiment 2
[0080] 1. The synthesis of compound 1-compound 3 and compound Boc-AMP-Glu-ene is as in Example 1.
[0081] 2. Synthesis of compound Boc-AMP-Glu-Gal
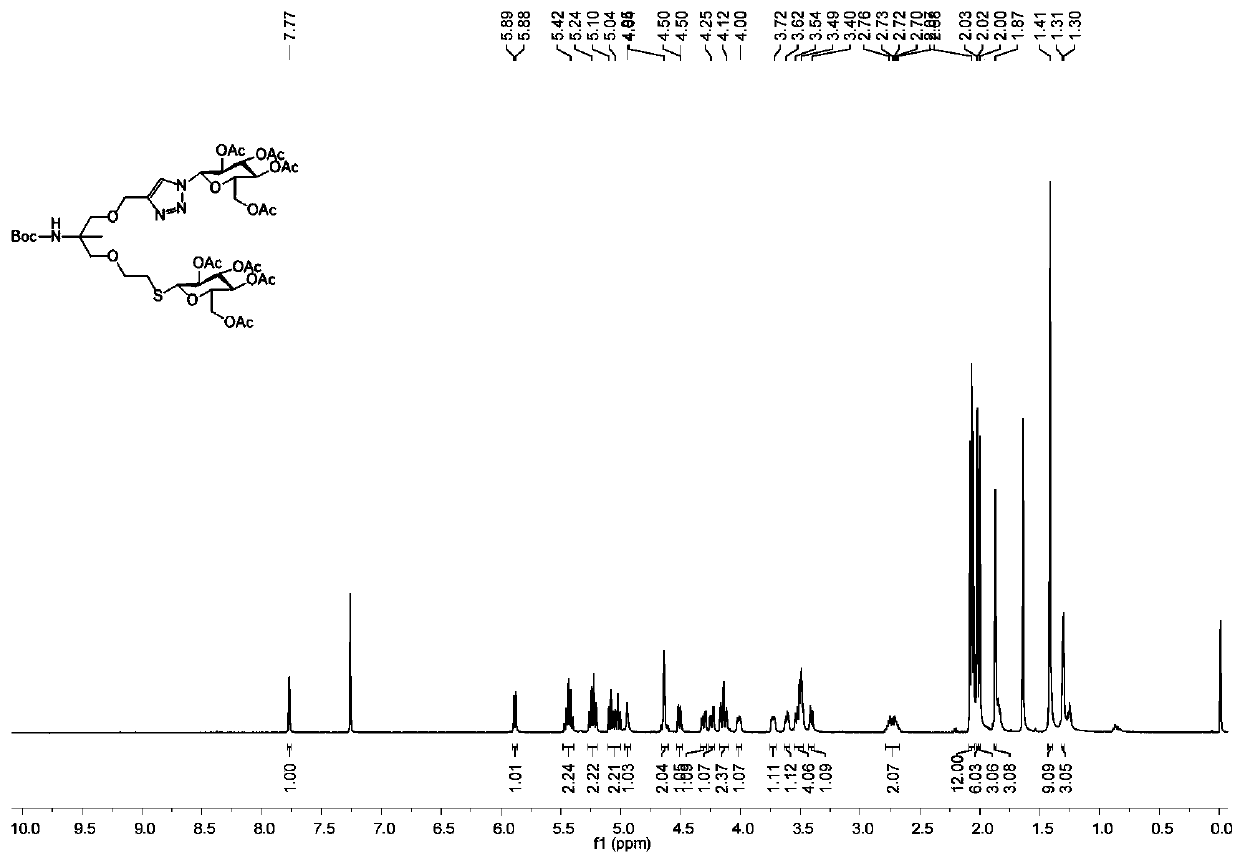
[0082] In the same synthesis method as Boc-AMP-Glu-Glu, the compound Boc-AMP-Glu-ene reacts with β-D-galactose sulfhydryl compound (Gal-SH) to prepare Boc-AMP-Glu-Gal, and obtains a colorless viscous material, yield 77%. The hydrogen and carbon spectra of Boc-AMP-Glu-Gal are as follows Figure 5 and Figure 6 shown. 1 H NMR (500MHz, CDCl 3 )δ=7.76(d, J=1.9Hz, 1H), 5.87(d, J=8.8Hz, 1H), 5.41(dt, J=12.1, 6.6Hz, 3H), 5.21(dt, J=16.2, 9.8 Hz, 2H), 5.03(dd, J=10.0, 3.3Hz, 1H), 4.93(s, 1H), 4.62(d, J=3.6Hz, 2H), 4.49(dd, J=10.0, 2.4Hz, 1H ),4.29(dd,J=12.6,4.9Hz,1H),4.15–4.06(m,3H),4.03–3.99(m,1H),3.94(t,J=6.6Hz,1H),3.60(dd, J=8.9, 4.3Hz, 1H), 3.50(ddd, J=12.7, 8.9, 4.2Hz, 4H), 3.40(d, J=8.5Hz, 1H), 2.72(ddd, J=17.4, 12.5, 5.6Hz ,2H),2.13(s,3H),2.08–2.03(m,9H),2.01(d,J=4.9Hz,6H),1.96(s,3H),1.85(s,3H),1.39(s, 9H), 1.28(d, J=2.8H...
Embodiment 3
[0084] 1. The synthesis of compound 1-compound 3 and compound Boc-AMP-Glu-ene is as in Example 1.
[0085] 2. Synthesis of compound Boc-AMP-Glu-Man
[0086] In the same synthesis method as Boc-AMP-Glu-Glu, the compound Boc-AMP-Glu-ene reacts with α-D-mannose sulfhydryl compound (Man-SH) to prepare Boc-AMP-Glu-Man, and obtains a colorless viscous material, yield 77%. The hydrogen and carbon spectra of Boc-AMP-Glu-Man are as follows Figure 7 and Figure 8 shown. 1 H NMR (500MHz, CDCl 3)δ=7.75(s,1H),5.87(d,J=8.8Hz,1H),5.46–5.40(m,2H),5.33–5.30(m,1H),5.27(d,J=9.8Hz,1H ),5.22(dd,J=10.6,7.1Hz,3H),4.89(s,1H),4.61(d,J=4.6Hz,2H),4.38–4.32(m,1H),4.32–4.27(m, 2H), 4.16–4.05(m, 4H), 4.03–3.99(m, 1H), 3.59(d, J=9.0Hz, 1H), 3.48–3.46(m, 2H), 3.40(d, J=6.6Hz ,1H),2.75–2.61(m,2H),2.14(s,3H),2.02(dd,J=12.8,7.1Hz,15H),1.97(s,3H),1.85(s,3H),1.39( s,9H),1.28(d,J=3.3Hz,3H). 13 C NMR (125MHz, CDCl 3 )δ=170.32,170.23,169.77,169.67,169.61,169.53,169.23,168.68,145.54,145.47,121.01,120.96,85...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com