Amlodipine besylate compound and novel preparation method thereof
A technology of amlodipine besylate and compounds, which is applied in the fields of organic chemistry and bulk chemical production, can solve the problems of low yield and achieve the effects of simplified reaction steps, high reaction yield and low price
Inactive Publication Date: 2010-08-25
HAINAN MEILAN SMITH KLINE PHARMA
View PDF3 Cites 11 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
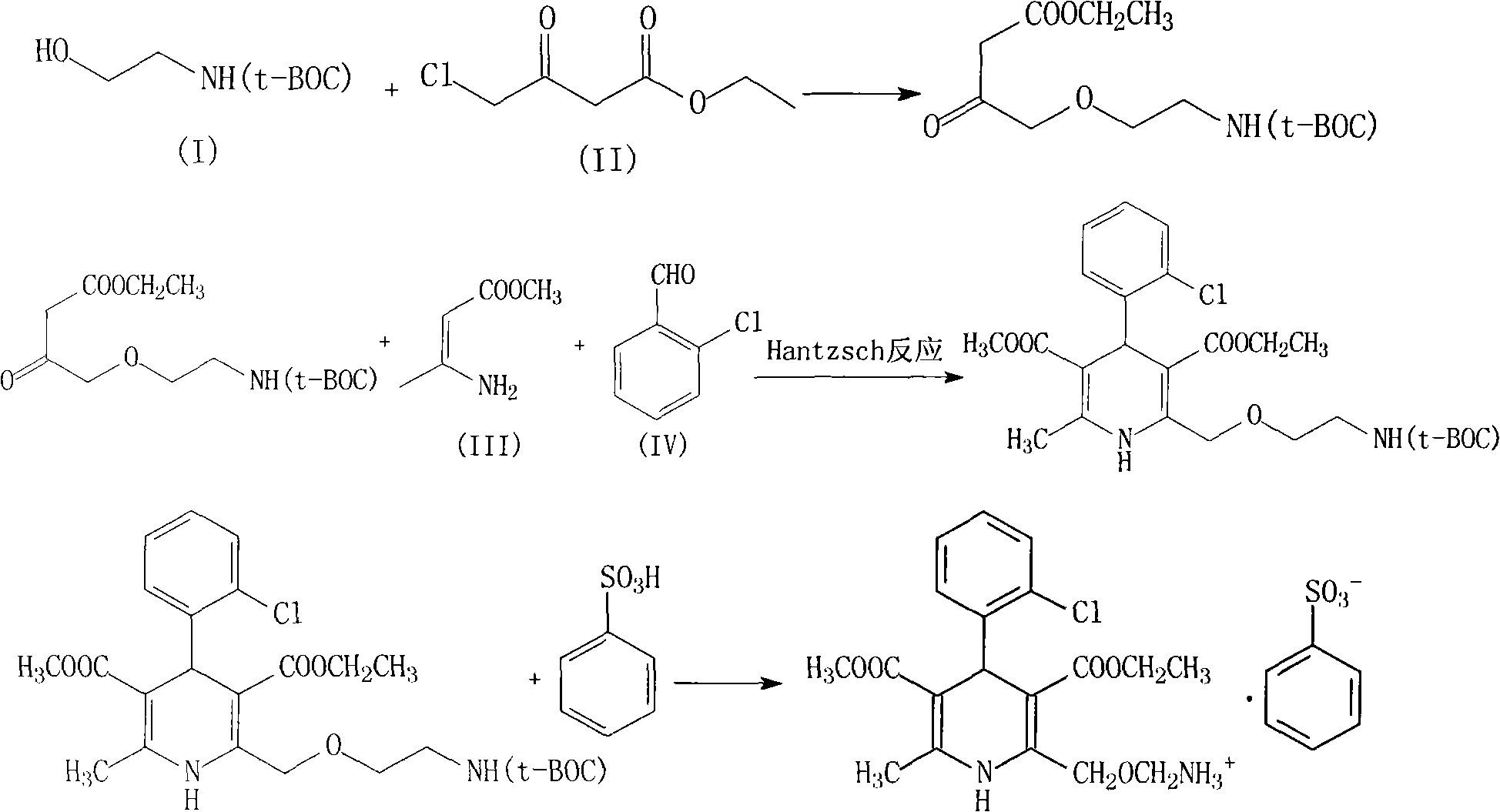
In order to overcome the low defect of the productive rate that occurs in the production of amlodipine besylate compound of the above-mentioned prior art, a large number of tests have been done, and the protection reagent di-tert-butyl dicarbonate (BOC) that adopts amino gentle protection reagent, through starting raw material tert-butyl Butoxycarbamide ethanol (HO(CH 2 ) 2 NH (t-BOC)) reacts with ethyl 4-chloroacetoacetate to introduce the Boc protecting group, and then reacts the intermediate 3-aminocrotonate methyl ester and 2-chlorobenzaldehyde to obtain amlodipine compound and benzene The direct reaction of sulfonic acid removes the BOC protecting group to obtain the final product
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
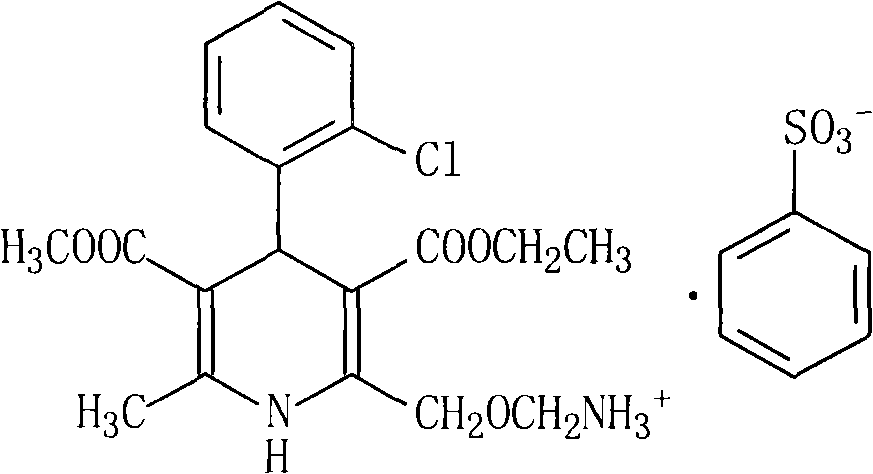
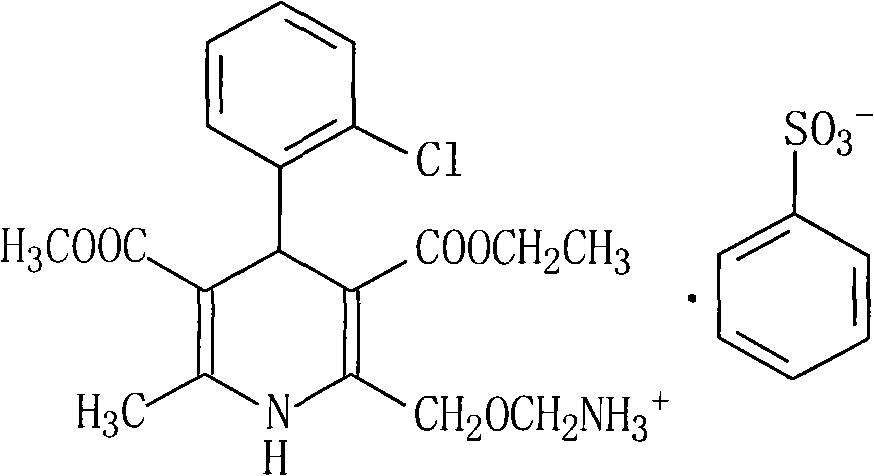
The invention relates to an amlodipine besylate compound and a novel preparation method thereof, wherein the method comprises the following steps that: a moderate amino-protecting reagent of di-tert-butyl dicarbonate is adopted so that the introduction of a Boc protecting group is realized, through the reaction of intermediums of 3-amino-crotonic acid methyl ester and 2-chlorobenzaldehyde, the obtained amlodipine besylate compound is directly carried out BOC protecting group removal reaction with benzene sulfonic acid, and the final product is obtained. Consequently, the invention is dispensed with the special deprotection step, simplifies the reaction steps, is more suitable for industrialized production and has high total reaction yield.
Description
technical field The invention relates to an amlodipine besylate compound and a new preparation method thereof, belonging to the technical field of medicine. Background technique Amlodipine besylate, chemical name: 3-ethyl-5-methyl-2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-1,4-dihydro- 6-Methyl-3,5-pyridinedicarboxylate benzenesulfonate, molecular formula: C 20 h 25 N 2 o 5 Cl·C 6 h 6 o 3 S, molecular weight: 567.1, structural formula: Amlodipine besylate is a third-generation dihydropyridine calcium antagonist developed by Pfizer of the United States. It is mainly used for the treatment of hypertension and angina. It was first launched in the UK in 1990, and its sales have increased year by year. The top selling product among ion antagonists. Compared with other similar drugs, this drug has three characteristics: (1) The blood pressure is stable and mild, and the incidence of adverse reactions is lower than that of conventional antagonists such as nifedipine; (2) ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D211/90
CPCY02P20/55
Inventor 王明
Owner HAINAN MEILAN SMITH KLINE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com