Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Suvorexant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat a certain sleep problem (insomnia).
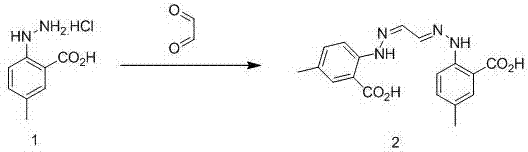
Synthetic method for anti-sleeplessness medicine MK-4305 intermediate
InactiveCN103012293ASave raw materialsSimple and fast operationOrganic chemistryCarboxyl radicalPhenylhydrazine hydrochloride
The invention belongs to the field of organic synthesis and in particular relates to a synthesis method for an anti-sleeplessness medicine MK-4305 (suvorexant) intermediate. According to the synthesis method provided by the invention, 4-methyl-2-carboxyl phenylhydrazine hydrochloride is taken as substrate, hydrazone formation, oxime formation, loop closing and deoxidization are carried out, and 2-(4-methyl-2-carboxyl phenyl)-1,2,3-triazole can be conveniently and effectively synthesized. Compared with the existing method, the synthesis method provided by the invention has the advantages that raw materials are low in price, operation is easy, industrial production is easy to realize and no isomer is formed, so that the synthesis method provided by the invention has important application value. The compound synthesized by adopting the invention is a key intermediate for synthesizing anti-sleeplessness medicine MK-4305 (suvorexant) medicinal molecules.
Owner:TONGJI UNIV
Novel routes of synthesis for preparation of suvorexant
InactiveCN106573882ACarbamic acid derivatives preparationOrganic compound preparationAntifungalOrganic chemistry
Owner:SANDOZ LTD
Synthesis method of suvorexant intermediate
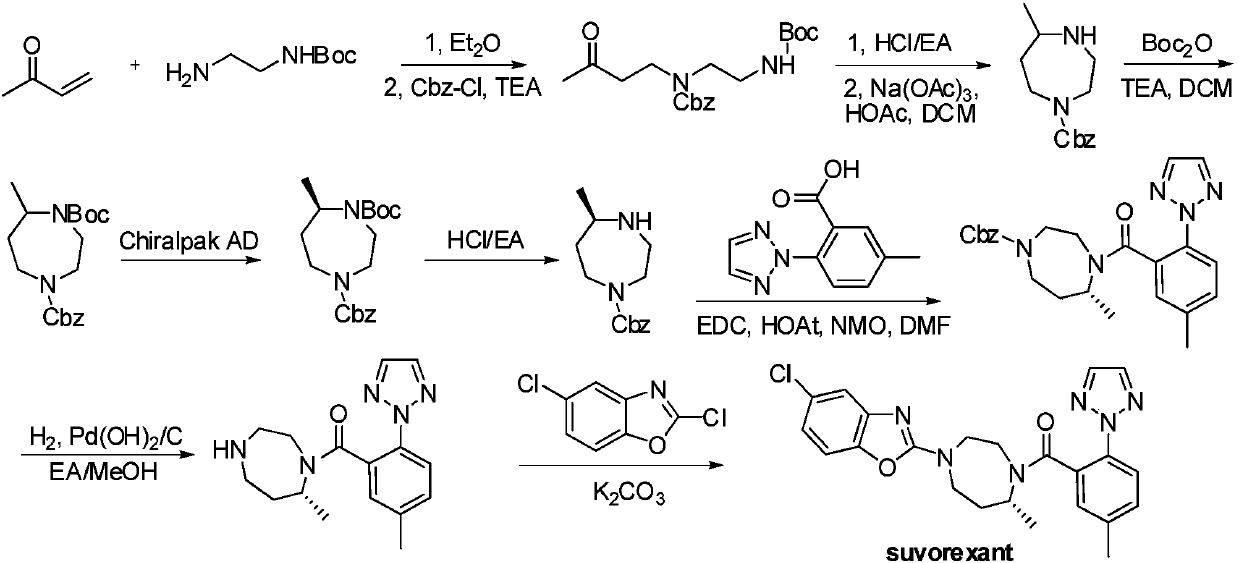
The invention provides a synthesis method of a suvorexant intermediate (5S)-hexahydro-5-methyl-1H-1,4-diazepine-1-carboxylic t-butyl ester. The synthesis method of the suvorexant intermediate (5S)-hexahydro-5-methyl-1H-1,4-diazepine-1-carboxylic t-butyl ester comprises the following steps: (1) enabling a compound Suvor-1 to react with vitride solution to obtain a compound Suvor-2; enabling the compound Suvor-2 to react with 4,4-dimethoxy-2-butanone to obtain Suvor-3; (3) enabling the compound Suvor-3 to react with methanesulfonic acid to obtain a compound Suvor-4; (4) enabling the compound Suvor-4 to react with di-tert-butyl dicarbonate to obtain a compound Suvor-5; (5) enabling the compound Suvor-5 to react with hydrogen to obtain the suvorexant intermediate. The synthesis method of the suvorexant intermediate (5S)-hexahydro-5-methyl-1H-1,4-diazepine-1-carboxylic t-butyl ester provided by the invention has the advantages that the reaction is simple, starting materials are cheap and easy to obtain, the reaction condition is mild, and the production safety is high; in addition, the reaction steps are less, the reaction yield is high, and the production cost is lower; moreover, by introducing a chiral functional group, the purity of a target product obtained after induced synthesis and ring closure is high, the amount of impurities in enantiomer is small, the ee% is greater than97%, and the method is suitable for commercial large-scale production.
Owner:成都美域高制药有限公司
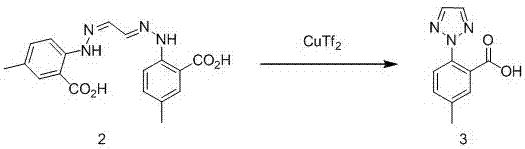
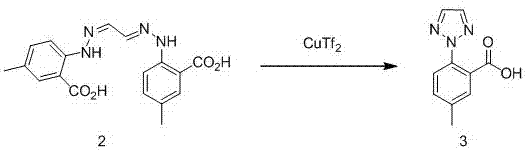
Synthetic method for intermediate of suvorexant as anti-insomnia medicament
The invention relates to a synthetic method for an intermediate of suvorexant as an anti-insomnia medicament. The synthetic method is characterized in that 4-methyl-2-hydrazinobenzoic acid hydrochloride is taken as a starting raw material, and reacts with glyoxal to generate dihydrazone; then dihydrazone is subjected to cyclization under the action of copper trifluoromethanesulfonate to obtain a key intermediate 5-methyl-2-(2H-1,2,3-triazole-2-yl) benzoic acid of the suvorexant as the anti-insomnia medicament. The synthetic method disclosed by the invention has the advantages of low price of raw materials, short reaction step, simpleness and convenience in operation, no isomers difficult to separate, high content of products and easiness for industrial production; meanwhile, a ring-closing by-product 4-methyl-2-carboxy anilide is separated in a form of forming hydrochloride, and is a raw materials for synthesizing the starting raw material 4-methyl-2-hydrazinobenzoic acid, so that organic materials can be recycled, and further the total synthetic cost is lower; in addition, by recovering treatment, solvent can be used indiscriminately, economy, environment friendliness and important application value are realized.
Owner:安徽联创生物医药股份有限公司
Preparation method of raw drug suvorexant
ActiveCN107298678AEasy separationHelp save energy and reduce consumptionOrganic chemistryAgent CombinationReaction temperature
The invention discloses a preparation method of raw drug suvorexant. The preparation method comprises the following step of generating S1 condensation acylation reaction by virtue of an intermediate I and an intermediate II in the presence of a condensing agent so as to generate an intermediate III, wherein the condensing agent is selected from one or combination of more than two of ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride, N,N'-carbonyldiimidazole, 1-hydroxybenzotriazole, 1-hydroxy-7-azobenzotriazole and tri(2,6-dimethoxyphenyl)bismuth. By adopting the proper condensing agent or condensing agent combination in the S1 condensation acylation reaction in the preparation method, and the yield in the prior art can be achieved at a reaction temperature of about 25 DEG C, and the condensing agent and intermediate products are simply separated, so that the energy saving and the consumption reduction in the large-scale industrial production are promoted.
Owner:安徽拜善晟制药有限公司
Novel Route of Synthesis for the Preparation of Suvorexant
InactiveUS20170217947A1Carbamic acid derivatives preparationOrganic compound preparationMedicinal chemistrySuvorexant
The present invention relates to a process for the preparation of a compound of formula (A), Further, the present invention relates to the respective compound (A) as such and to its use in the preparation of antifungal agent.
Owner:SANDOZ AG
Method for preparing important Suvorexant intermediate
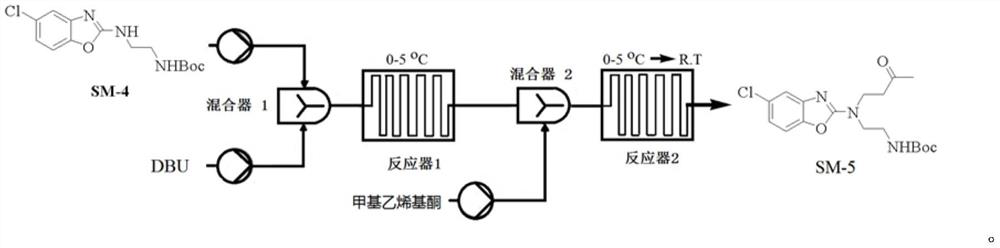
The invention belongs to the technical field of medicines and relates to a method for preparing an important intermediate {2-[(5-chlorobenzoxazole-2-yl)-(3-one-butyl)-amino]ethyl}-tert-butyl carbamate. A highly toxic product methyl vinyl ketone is substituted by a compound with low toxicity, and due to catalytic reactions of DBU, LDA, NaHMDS, KHMDS and the like, a compound of a formula (3) can be obtained at high yield of 90% or higher. Moreover, the HPLC (high performance liquid chromatography) content of the crude product reaches 99.5% or higher, and the method disclosed by the invention is suitable for industrial production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Method for preparing Suvorexantintermediate and analogue thereof
InactiveCN106866632ASolve usabilitySolve the costOrganic chemistryBulk chemical productionHydrogenToxic material
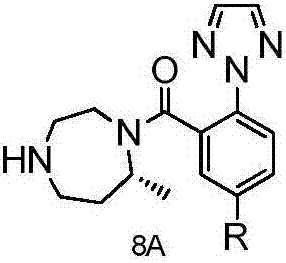
The invention discloses a method for preparing a Suvorexantintermediate as shown in a formula 8A and an analogue thereof, or pharmaceutically acceptable salts and solvates thereof, wherein R is hydrogen or an alkyl group of C1 to C6. The method comprises the following steps of removing an amino protection group of a compound as shown in a formula 3 to obtain a compound as shown in a formula 4A; d, adopting the compound as shown in the formula 4A for preparing to obtain a compound as shown in a formula 5A; e, adopting the formula 5A for preparing to obtain a compound as shown in a formula 6A; f, reacting the compound as shown in the formula 6A and a compound as shown in a formula 10A for preparing to obtain a compound as shown in a formula 7A; g, adopting the compound as shown in the formula 7A for preparing to obtain the compound as shown in the formula 8A. According to the method provided by the invention, the compound 8 is synthesized through a brand new process route, and is then adopted as an intermediate for preparing Suvorexant, so that the problems of the usage of toxic substances, high cost, long route and low yield in an existing method for preparing the Suvorexant are effectively solved, and the method is suitable for industrial application.
Owner:成都美域高制药有限公司
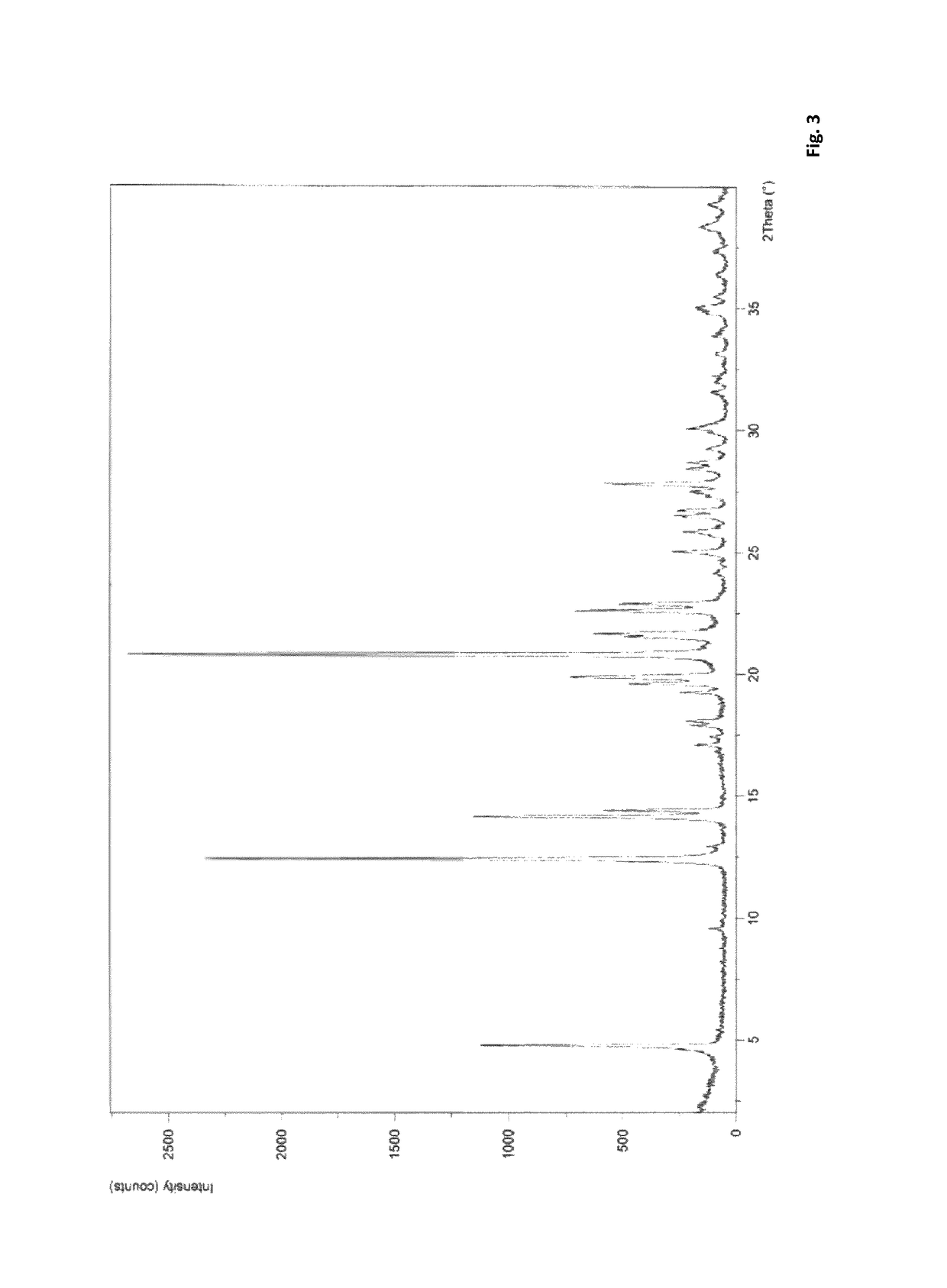
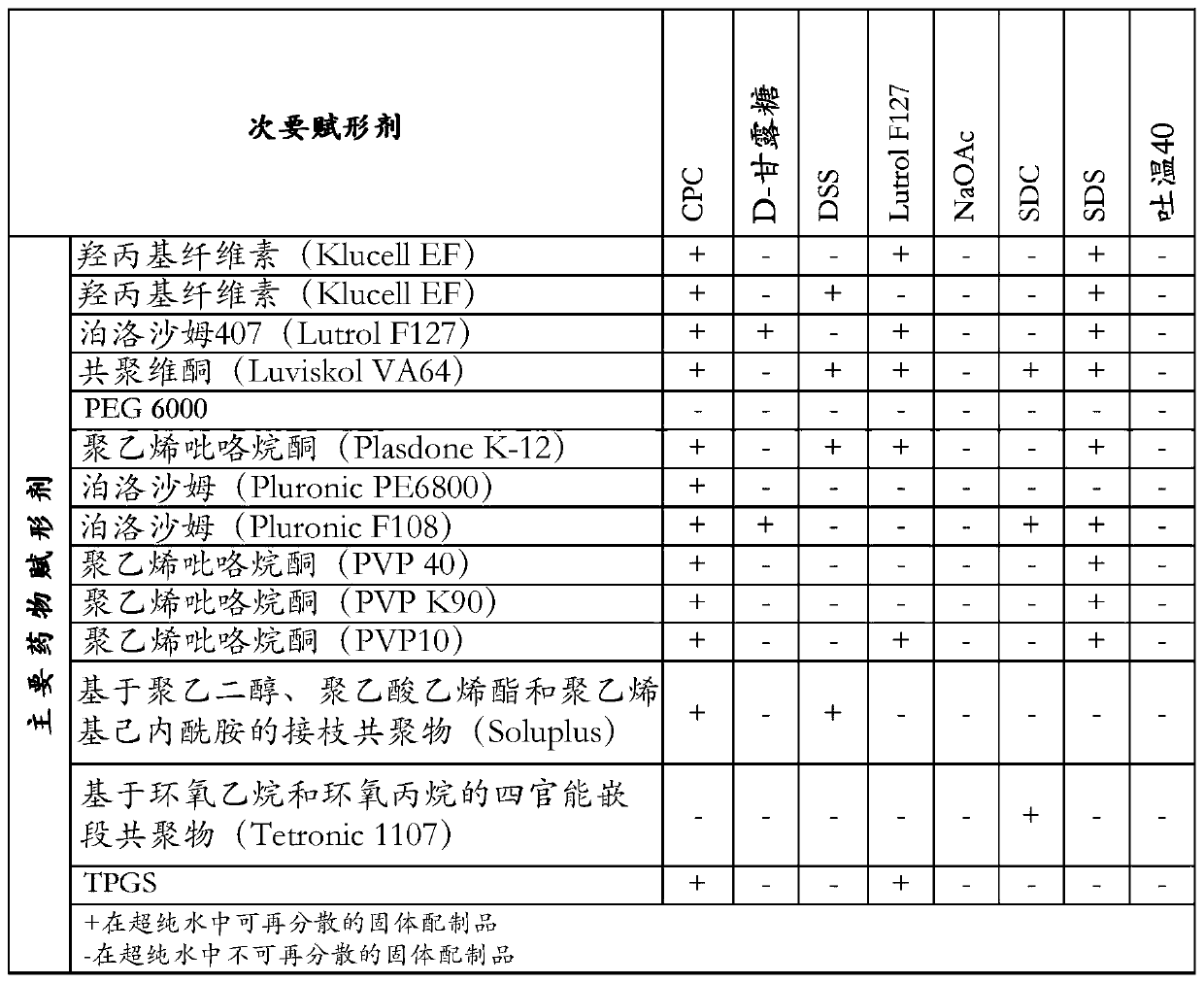
Solid Dispersion Comprising An Orexin Receptor Antagonist
InactiveUS20170027873A1Good dispersionImprove long-term stabilityOrganic active ingredientsNervous disorderOREXIN A RECEPTOROrexin Receptor Antagonists
A solid dispersion comprising suvorexant or a salt thereof in amorphous form and at least one pharmaceutically acceptable matrix compound, wherein the matrix compound is (i) a polymer and wherein the solid dispersion contains the suvorexant or salt thereof in an 5 amount of at least 50 weight-% based on the combined weight of the suvorexant or salt thereof and the at least one matrix compound, or (ii) a silicon-based inorganic adsorbent.
Owner:SANDOZ AG
Suvorexant intermediate preparation method
InactiveCN107935946AThe reaction steps are simpleAvoid handlingCarbamic acid derivatives preparationOrganic compound preparationEconomic benefitsSafe operation
The invention relates to a preparation method of a suvorexant intermediate represented by a formula IV, wherein the preparation route is defined in the specification. According to the present invention, the method has advantages of simple and safe operation, good yield, low environmental pollution and good economic benefits, and is suitable for industrial production. The formulas III and IV are defined in the specification, wherein R represents C1-5 substituted or unsubstituted benzyl and allyl.
Owner:SHANGHAI SYNCORES TECH INC
Route of synthesis for the preparation of suvorexant
InactiveUS10030010B2Carbamic acid derivatives preparationOrganic compound preparationMedicinal chemistrySuvorexant
The present invention relates to a process for the preparation of a compound of formula (A), Further, the present invention relates to the respective compound (A) as such and to its use in the preparation of antifungal agent.
Owner:SANDOZ AG
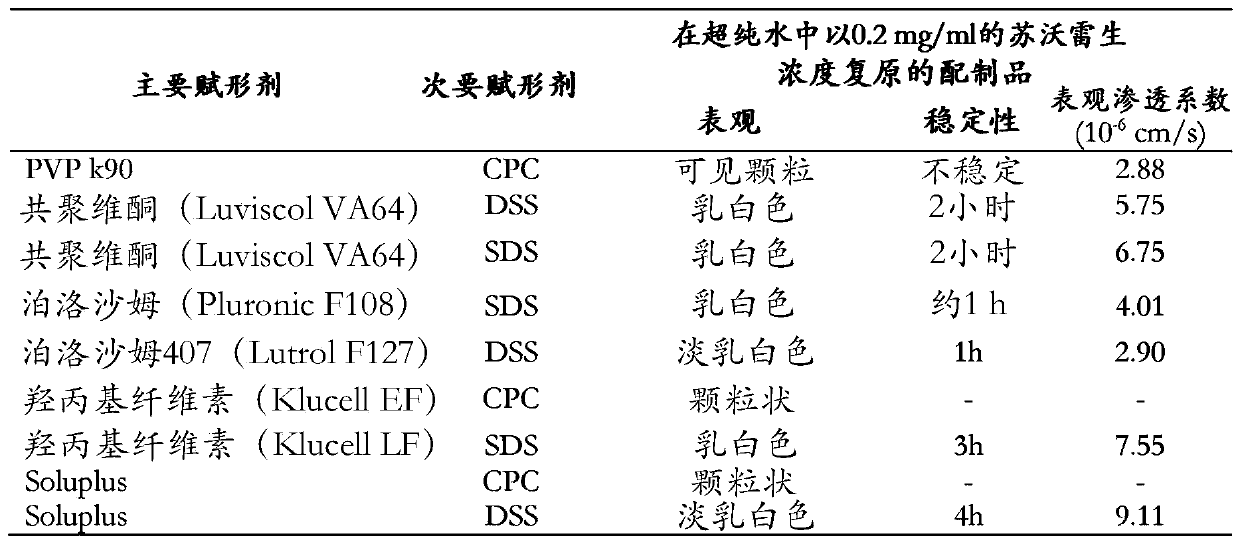
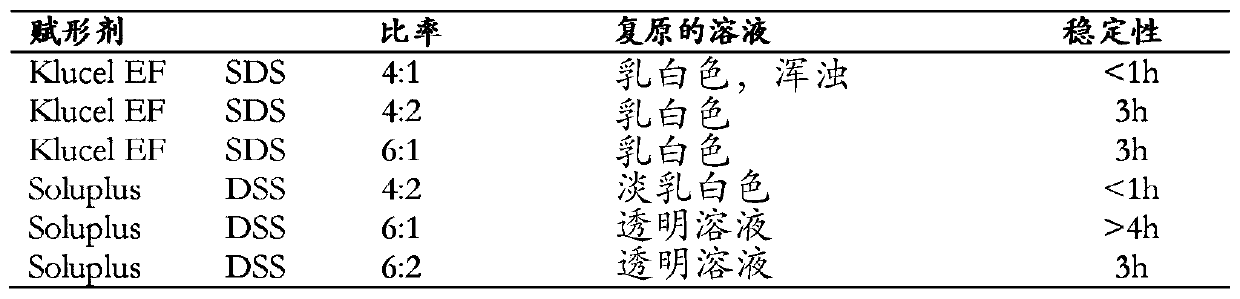
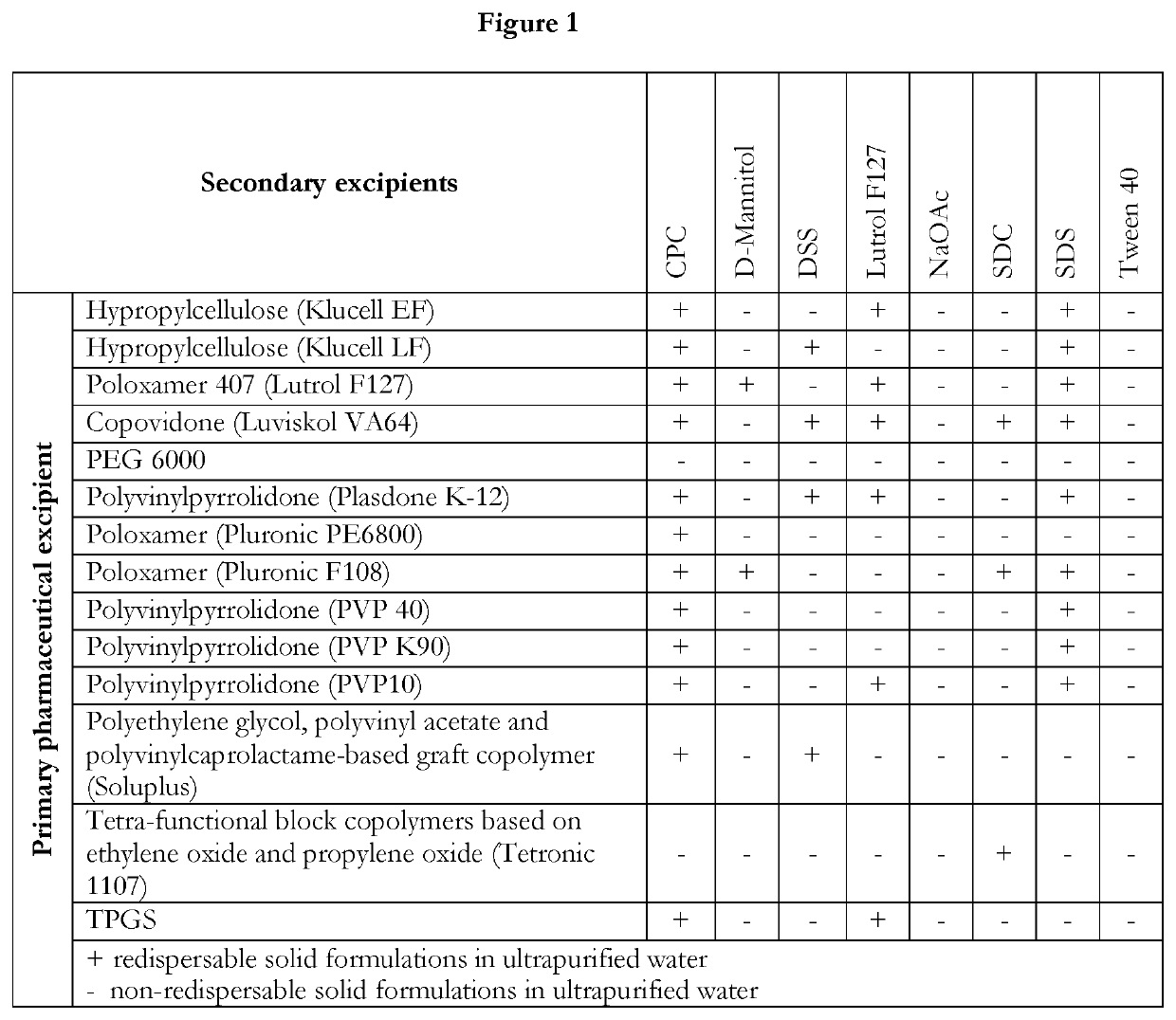
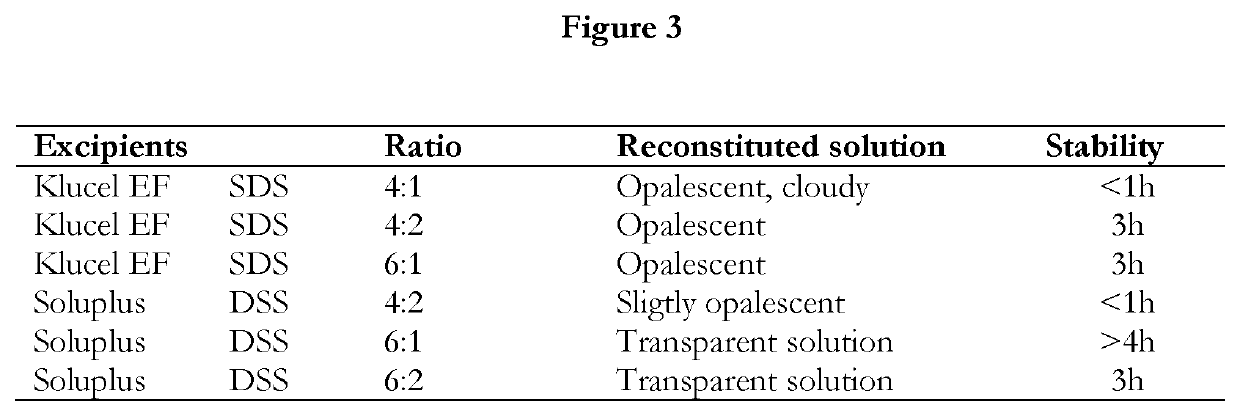
Pharmaceutical formulations of suvorexant
InactiveCN109996548AEasy to predictImprove solubilityOrganic active ingredientsPowder deliveryMetaboliteFOOD EFFECT
The present invention relates to pharmaceutical formulations comprising as active compound Suvorexant, or its salts, or its metabolites or derivatives, thereof and pharmaceutical excipients, process for the preparation thereof and pharmaceutical compositions containing them. The pharmaceutical formulations of the present invention possess instantaneous redispersibility, increased apparent solubility and permeability, no observable food effect with respect to immediate absorption and more predictable plasma concentration throughout the night and next morning. The invention also relates to methods of manufacturing the pharmaceutical formulations and pharmaceutical compositions containing them according to the invention, their uses and methods of treatments using the pharmaceutical formulations and their pharmaceutical compositions.
Owner:DRUGGABILITY TECH IP HOLDCO
Suvorexant intermediate and preparation method thereof
ActiveUS10618891B2Atom utilization is highHigh production costCarbamic acid derivatives preparationOrganic compound preparationEthyl groupCombinatorial chemistry
The present invention relates to a synthesis process of suvorexant, novel compounds represented by formulas II, III, IV or V, or salts thereof for preparing suvorexant, and a method for preparing the intermediates. The preparation method uses a chiral starting material to synthesize chiral compounds represented by formulas II, III, IV or V, the compounds obtained being used for synthesizing the suvorexant. The preparation method has the advantages of simple operation, low cost, mild reaction conditions, simple post-treatment, easy to purify, high yield, high ee value for the product, and easy to industrialize. In the reaction route shown, R represents benzyl, allyl or 1-phenethyl, or optionally substituted benzyl at the 2 position to 6 position, such as 4-methoxybenzyl, 4-nitrobenzyl, 2-methylbenzyl, 4-chlorobenzyl or 3-fluorobenzyl.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +1
Chiral resolution of an intermediate of suvorexant and cocrystals thereof
InactiveUS20190276414A1Promote recoverySatisfactory yieldNervous disorderOptically-active compound separationCarboxylic saltPhenyl group
Relating to processes for preparing suvorexant or its pharmaceutically acceptable salts through the formation of a cocrystal of (R)-benzyl 5-methyl-1,4-diazepane-1-carboxylate hydrochloride with (R)-(+)-1,1,2-triphenyl-1,2-ethanediol ((R)-TED). This cocrystal provides the resolution of an intermediate of suvorexant, in particular, of (rac)-benzyl5-methyl-1,4-diazepane-1-carboxy-lateor a hydrochloride salt thereof. It also relates to a new cocrystal useful in such preparation processes.
Owner:ENANTIA
A kind of preparation method of raw material Suvorexan
ActiveCN107298678BEasy separationHelp save energy and reduce consumptionOrganic chemistryEthyl groupReaction temperature
The invention discloses a preparation method of raw drug suvorexant. The preparation method comprises the following step of generating S1 condensation acylation reaction by virtue of an intermediate I and an intermediate II in the presence of a condensing agent so as to generate an intermediate III, wherein the condensing agent is selected from one or combination of more than two of ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride, N,N'-carbonyldiimidazole, 1-hydroxybenzotriazole, 1-hydroxy-7-azobenzotriazole and tri(2,6-dimethoxyphenyl)bismuth. By adopting the proper condensing agent or condensing agent combination in the S1 condensation acylation reaction in the preparation method, and the yield in the prior art can be achieved at a reaction temperature of about 25 DEG C, and the condensing agent and intermediate products are simply separated, so that the energy saving and the consumption reduction in the large-scale industrial production are promoted.
Owner:安徽拜善晟制药有限公司
A kind of Suwo Lexan intermediate and preparation method thereof
ActiveCN111943945BReduce usageAvoid separationCarbamic acid derivatives preparationOrganic compound preparationEthyl groupCombinatorial chemistry
The invention discloses a Suvorexan intermediate and a preparation method thereof. Said Suvorexan intermediate is wherein, R represents methyl or ethyl, and PG1 and PG2 represent amino protecting groups. The preparation method is as follows: carrying out a Michael addition reaction with the compound III to obtain compound III; removing the amino protecting group from compound III to generate compound IV; reducing compound IV to obtain compound V; protecting the secondary amine in compound V with an amino protecting group to obtain compound IV VI. The invention avoids the use of the highly toxic compound methyl vinyl ketone, obtains the intermediate of Suvorexan in the desired configuration by using the chiral starting material, and avoids the use of a chiral resolving agent or separation by chiral HPLC , as well as avoiding the use of transition metals and biological enzyme preparations for chiral catalysis. The reaction conditions have the advantages of simple post-processing, easy separation and purification, high yield, high ee value, and easy industrialization.
Owner:SHANGHAI INST OF TECH
Continuous flow micro-reaction synthesis method of suvorexant intermediate
PendingCN113929673ASolve volatileAddresses highly toxic propertiesOrganic chemistryEthyl groupHalogenation
The invention relates to a continuous flow micro-reaction synthesis method of a suvorexant intermediate, and belongs to the technical field of medicine preparation. According to the preparation method, a key intermediate of a suvorexant bulk drug is technically optimized by adopting a continuous flow microreactor technology, and the suvorexant intermediate is synthesized by taking 2-amino-4-chlorophenol and potassium ethyl xanthate as initial raw materials through cyclization reaction, halogenation reaction, chiral resolution and other reactions. Meanwhile, influences of halogenating reagent types, acid-binding agent types and alkali equivalent factors involved in the technological process on the yield and purity of corresponding intermediates are investigated, and the optimal technological conditions are determined. The optimization process has the advantages of mild reaction conditions, simplicity and convenience in purification, environmental friendliness and the like, and is suitable for industrial large-scale production.
Owner:湖南华腾医药有限公司
Synthesis method of anti-insomnia drug Suvorexan intermediate
The invention relates to a synthetic method for an intermediate of suvorexant as an anti-insomnia medicament. The synthetic method is characterized in that 4-methyl-2-hydrazinobenzoic acid hydrochloride is taken as a starting raw material, and reacts with glyoxal to generate dihydrazone; then dihydrazone is subjected to cyclization under the action of copper trifluoromethanesulfonate to obtain a key intermediate 5-methyl-2-(2H-1,2,3-triazole-2-yl) benzoic acid of the suvorexant as the anti-insomnia medicament. The synthetic method disclosed by the invention has the advantages of low price of raw materials, short reaction step, simpleness and convenience in operation, no isomers difficult to separate, high content of products and easiness for industrial production; meanwhile, a ring-closing by-product 4-methyl-2-carboxy anilide is separated in a form of forming hydrochloride, and is a raw materials for synthesizing the starting raw material 4-methyl-2-hydrazinobenzoic acid, so that organic materials can be recycled, and further the total synthetic cost is lower; in addition, by recovering treatment, solvent can be used indiscriminately, economy, environment friendliness and important application value are realized.
Owner:安徽联创生物医药股份有限公司
A kind of synthetic method of Suwo Leisheng intermediate
The present invention provides a method for synthesizing Suvorexan intermediate (5S)-hexahydro-5-methyl-1H-1,4-diazepine-1-carboxylate tert-butyl ester, comprising the following steps: (1) compound Suvor-1 reacts with red aluminum solution to obtain compound Suvor-2; (2) compound Suvor-2 reacts with 4,4-dimethoxy-2-butanone to obtain Suvor-3; (3) ) compound Suvor-3 reacts with methanesulfonic acid to obtain compound Suvor-4; (4) compound Suvor-4 reacts with di-tert-butyl dicarbonate to obtain compound Suvor-5; (5) compound Suvor-5 reacts with hydrogen, Obtain Suvorexan intermediates. The method has simple reaction, cheap and easy-to-obtain starting materials, mild reaction conditions, and high production safety; in addition, the method has few reaction steps, high reaction yield, and low production cost; Induced synthesis, the target product obtained after ring closure has high purity, less enantiomeric impurities, and ee% is greater than 97%, which is suitable for commercial scale production.
Owner:成都美域高制药有限公司
Pharmaceutical formulations of suvorexant
InactiveUS20200078303A1Improved physicochemical characteristicImprove biological performanceOrganic active ingredientsPowder deliveryMetaboliteFOOD EFFECT
The present invention relates to pharmaceutical formulations comprising as active compound Suvorexant, or its salts, or its metabolites or derivatives, thereof and pharmaceutical excipients, process for the preparation thereof and pharmaceutical compositions containing them. The pharmaceutical formulations of the present invention possess instantaneous redispersibility, increased apparent solubility and permeability, no observable food effect with respect to immediate absorption and more predictable plasma concentration throughout the night and next morning. The invention also relates to methods of manufacturing the pharmaceutical formulations and pharmaceutical compositions containing them according to the invention, their uses and methods of treatments using the pharmaceutical formulations and their pharmaceutical compositions.
Owner:NANGENEX NANOTECH
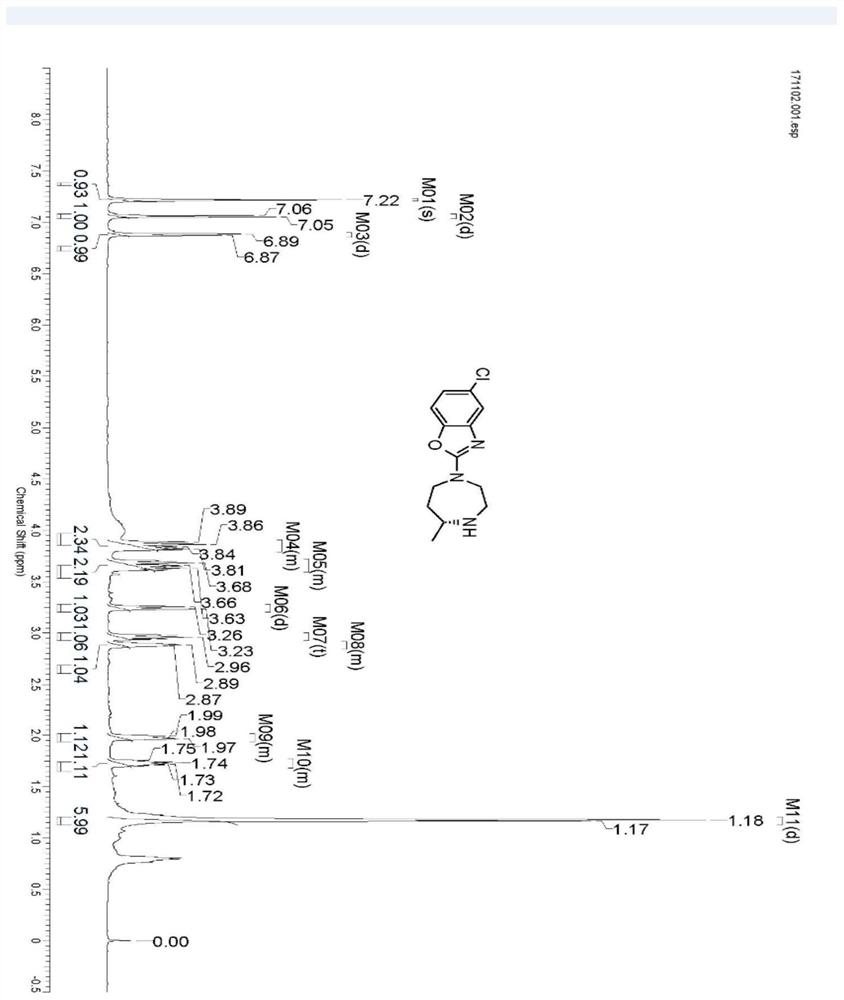
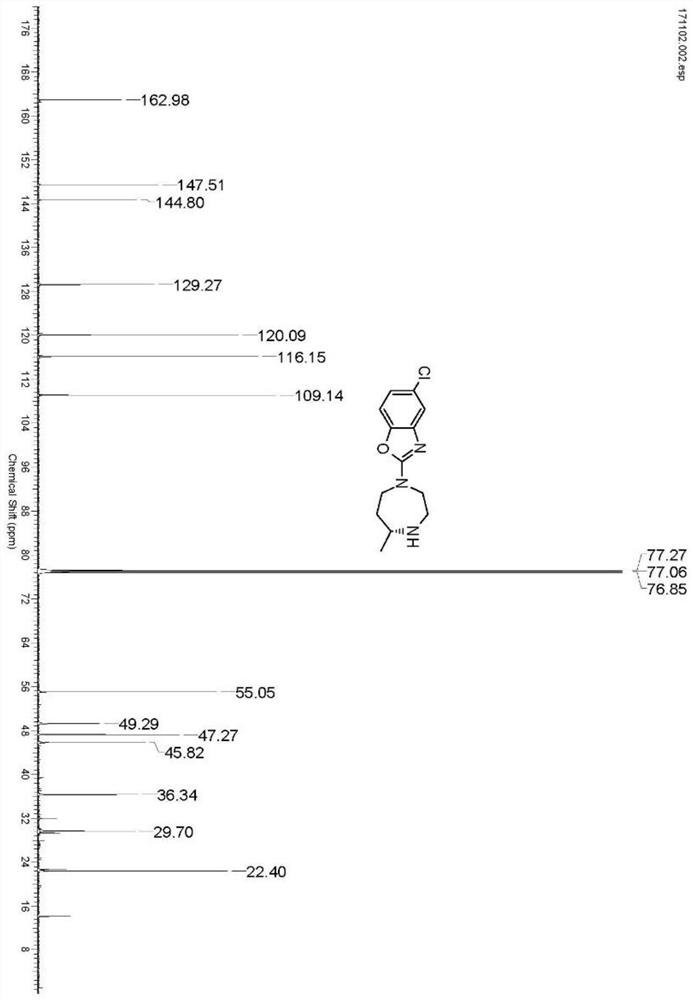
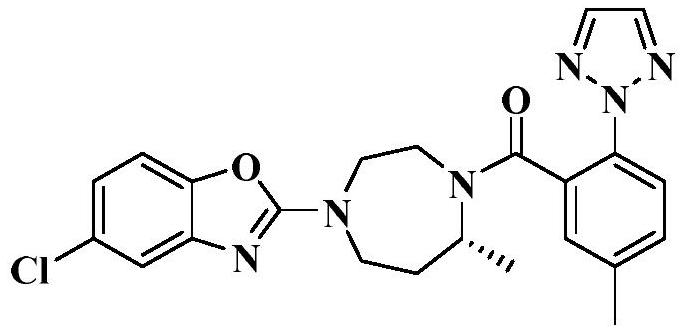
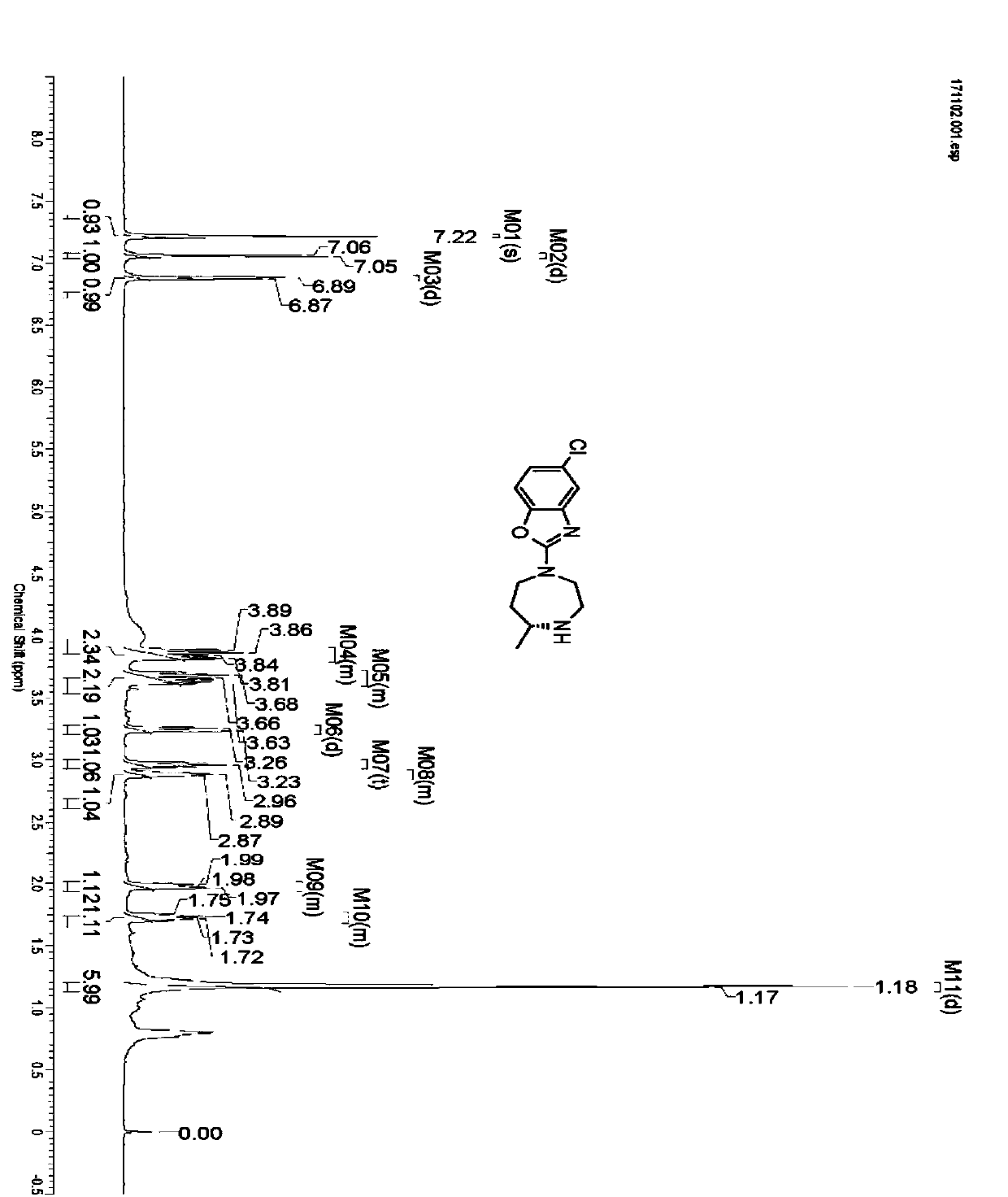
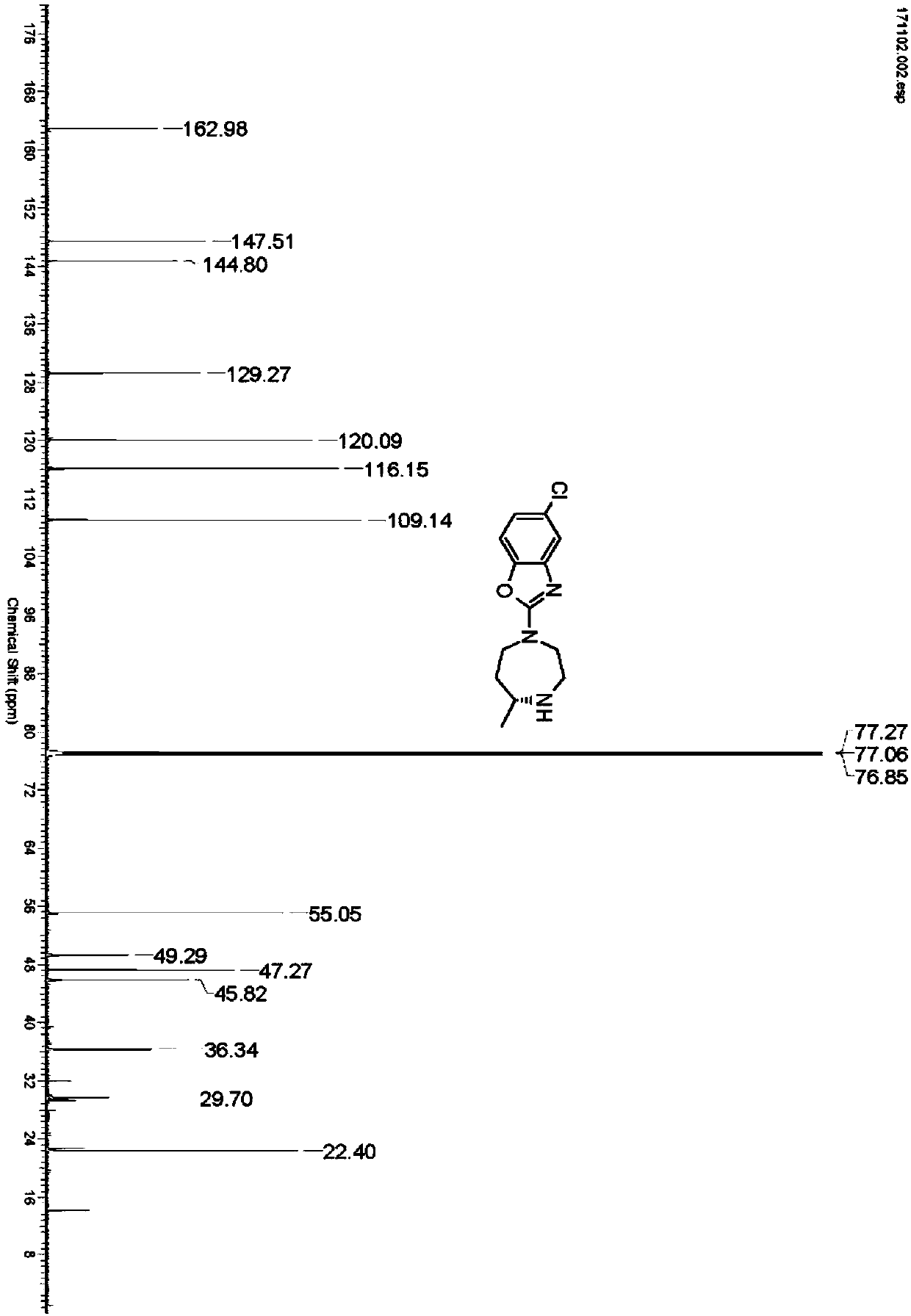
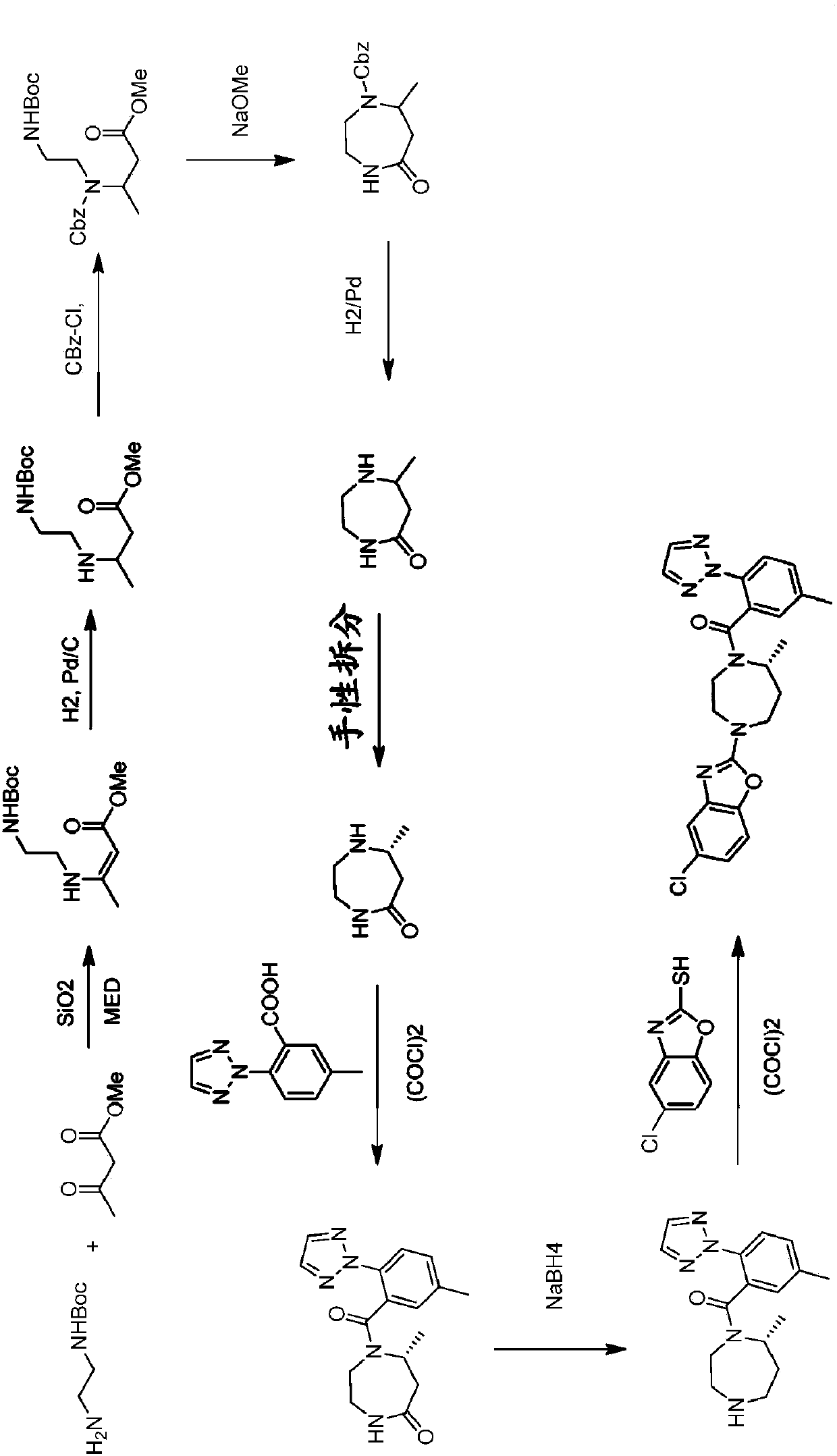
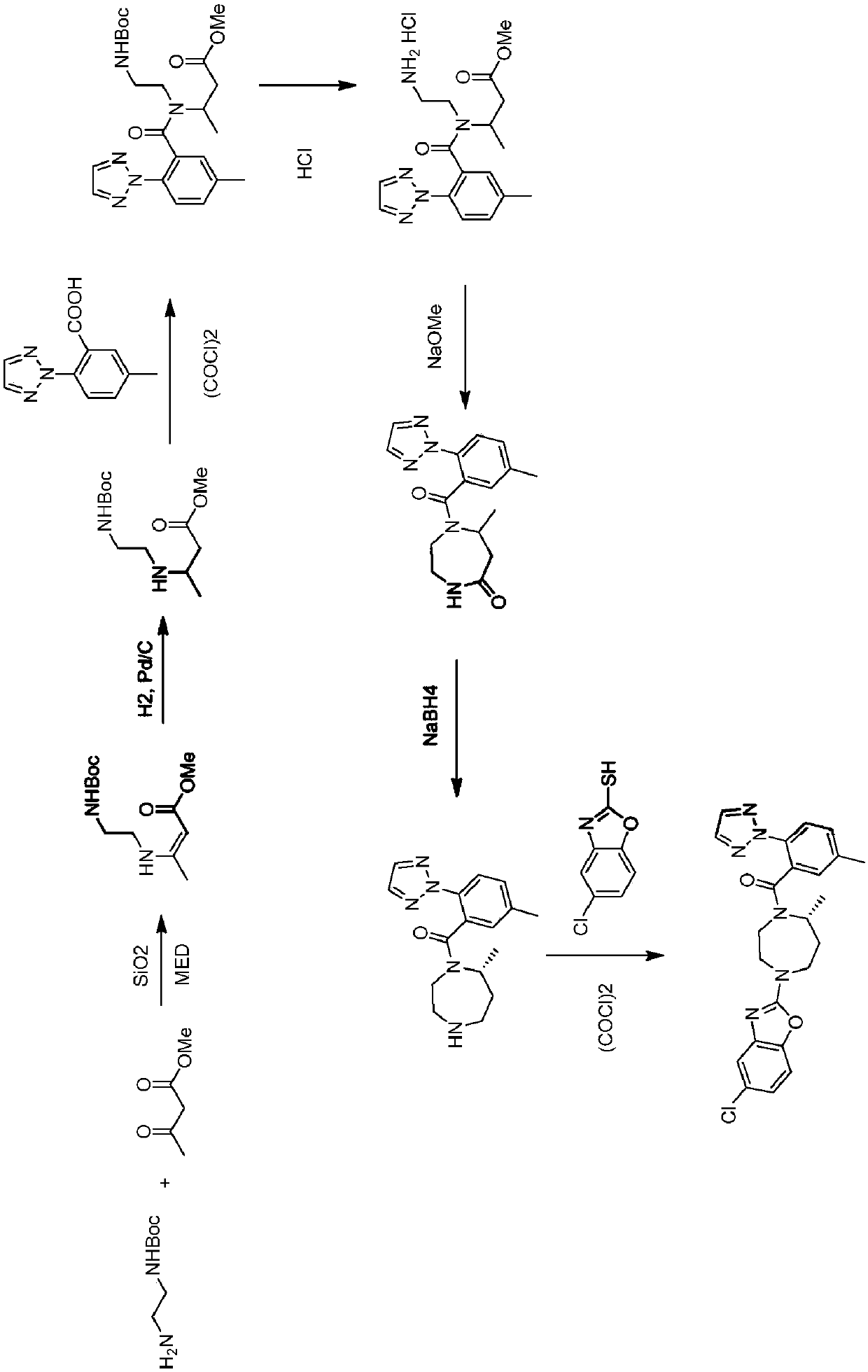
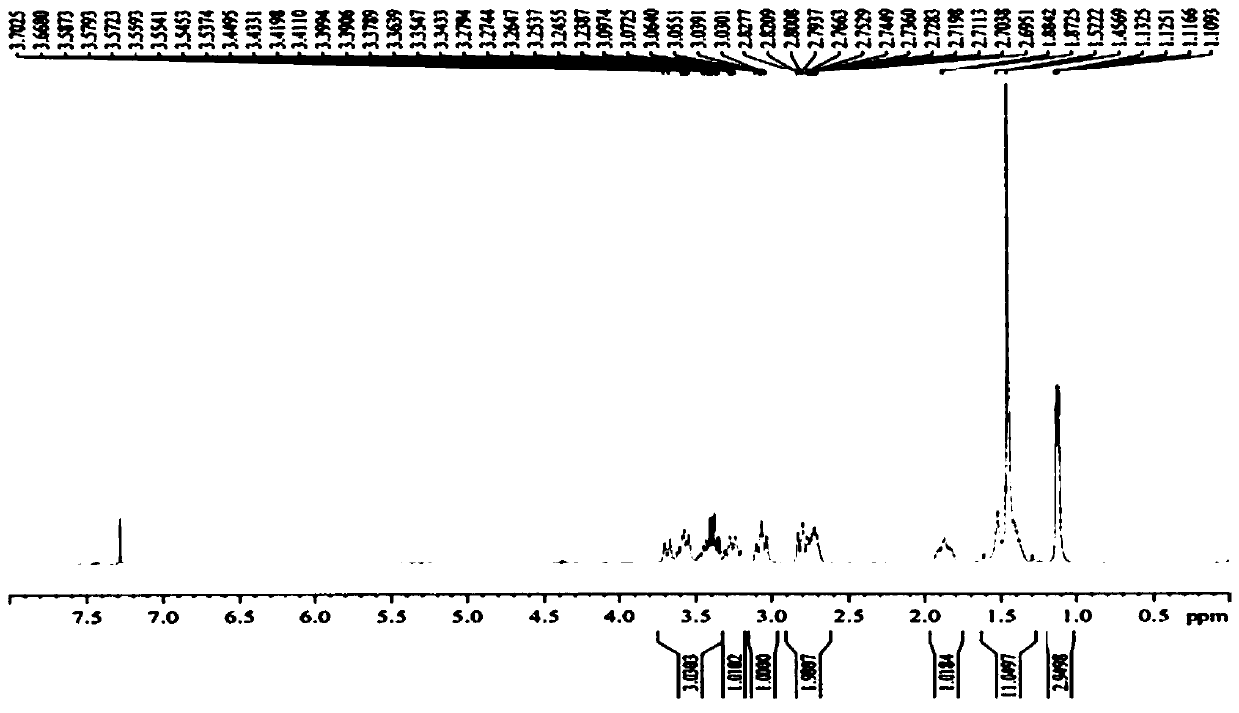
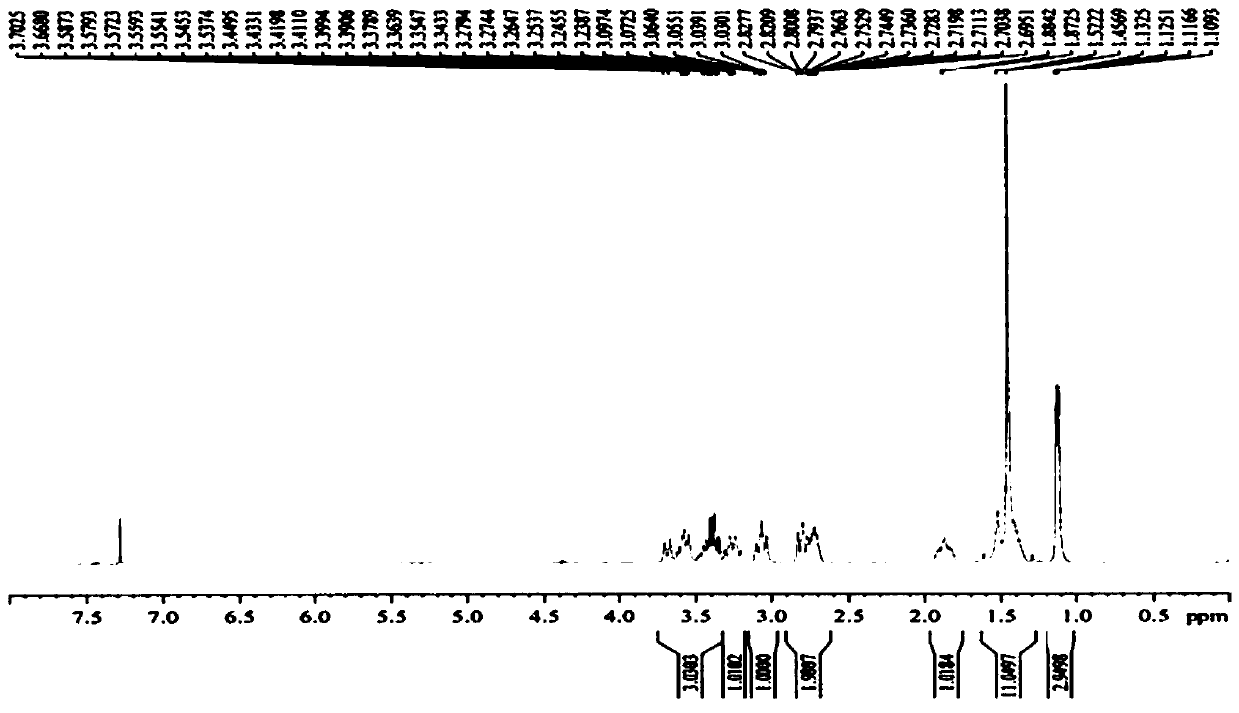
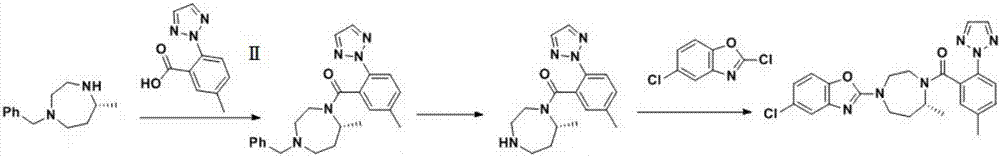
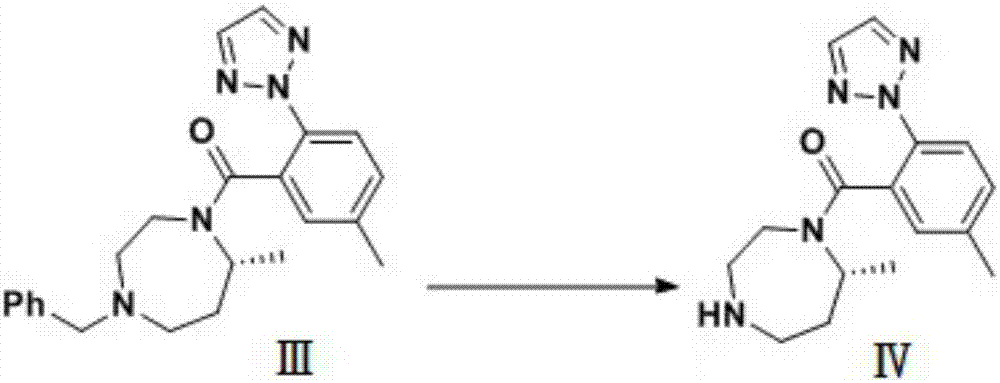
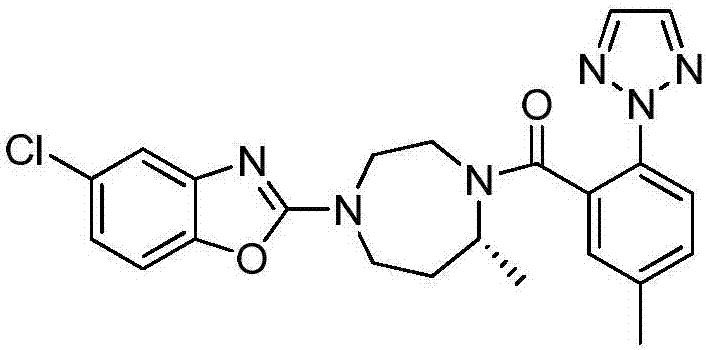
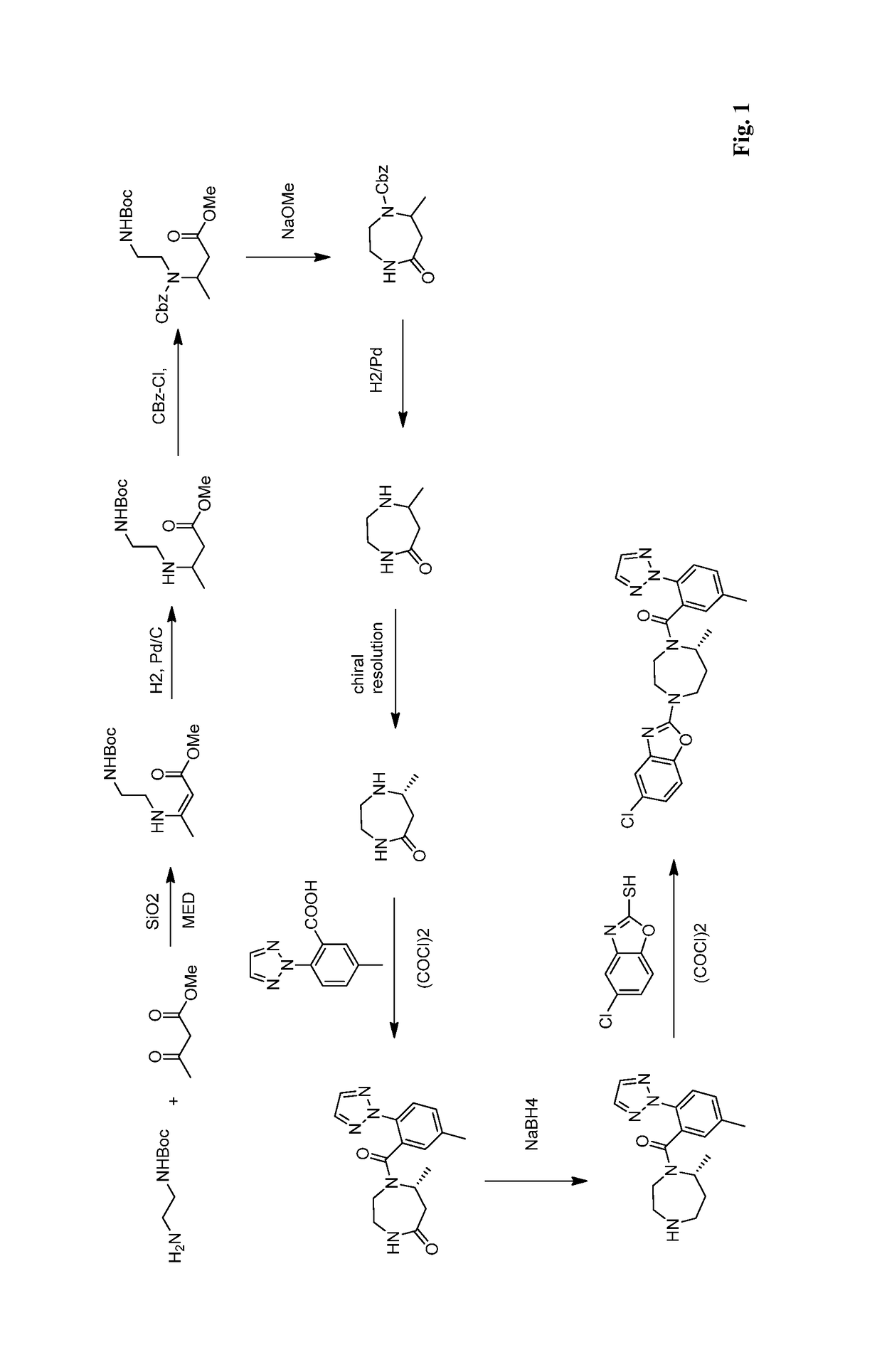
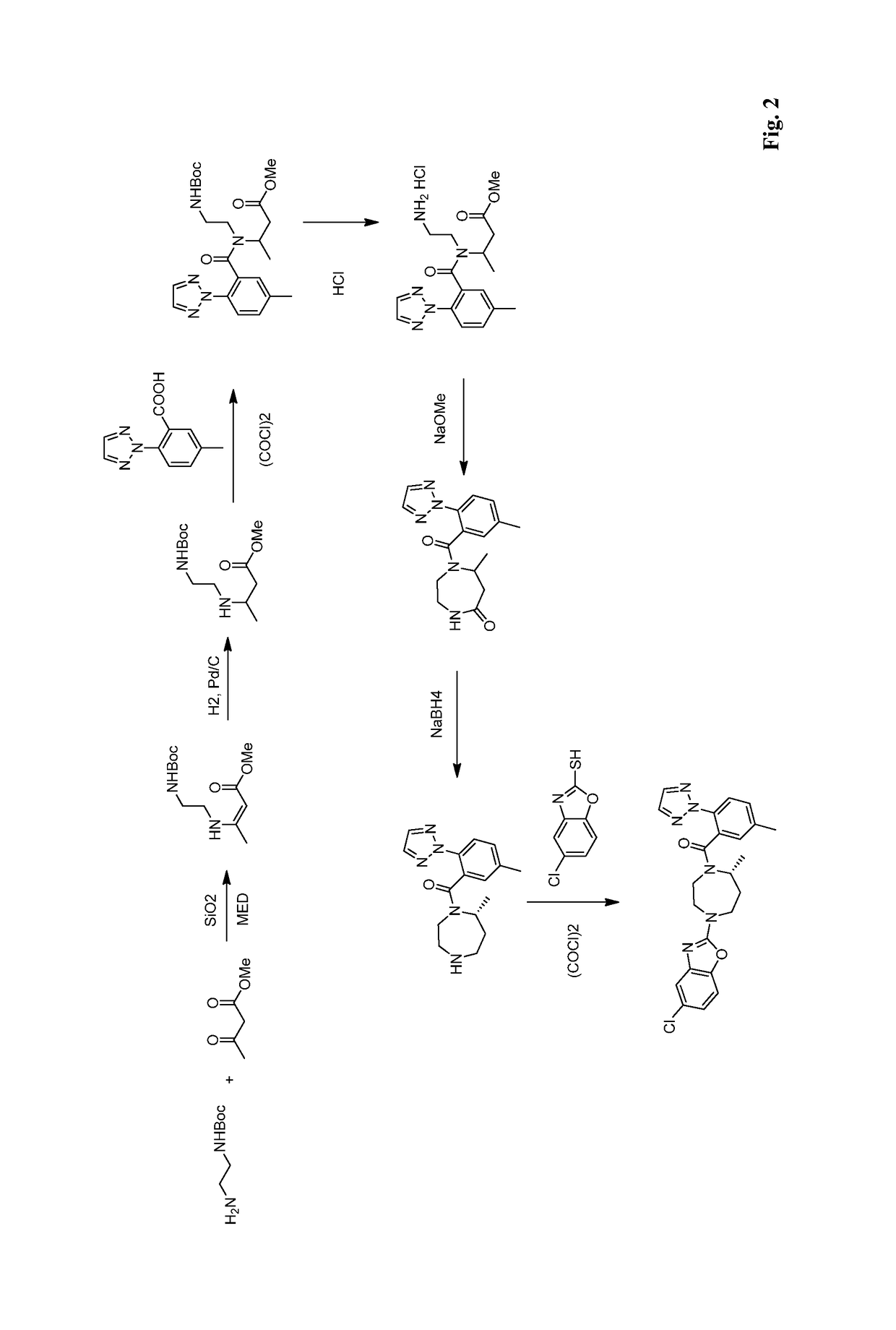
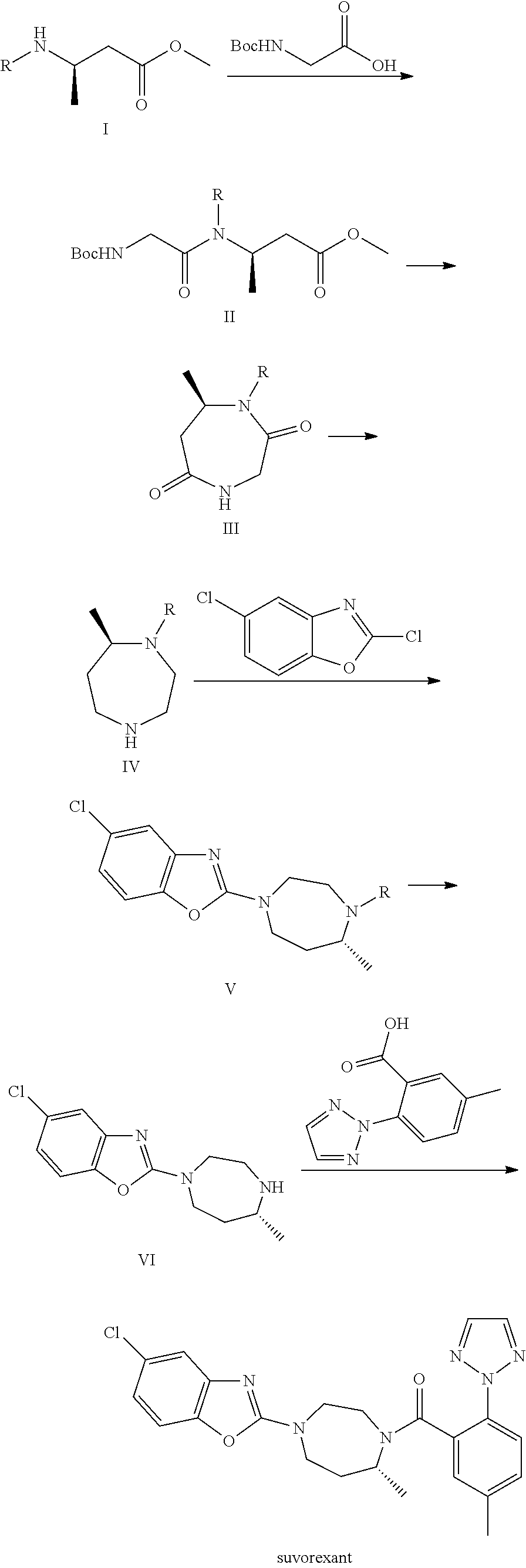
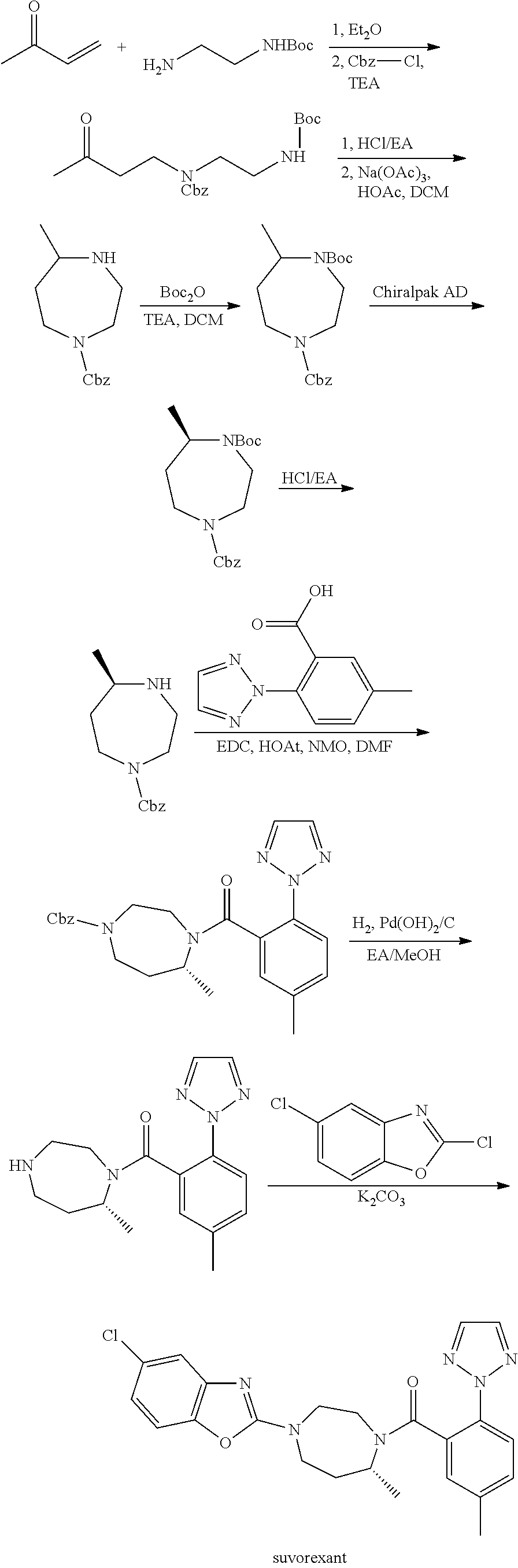
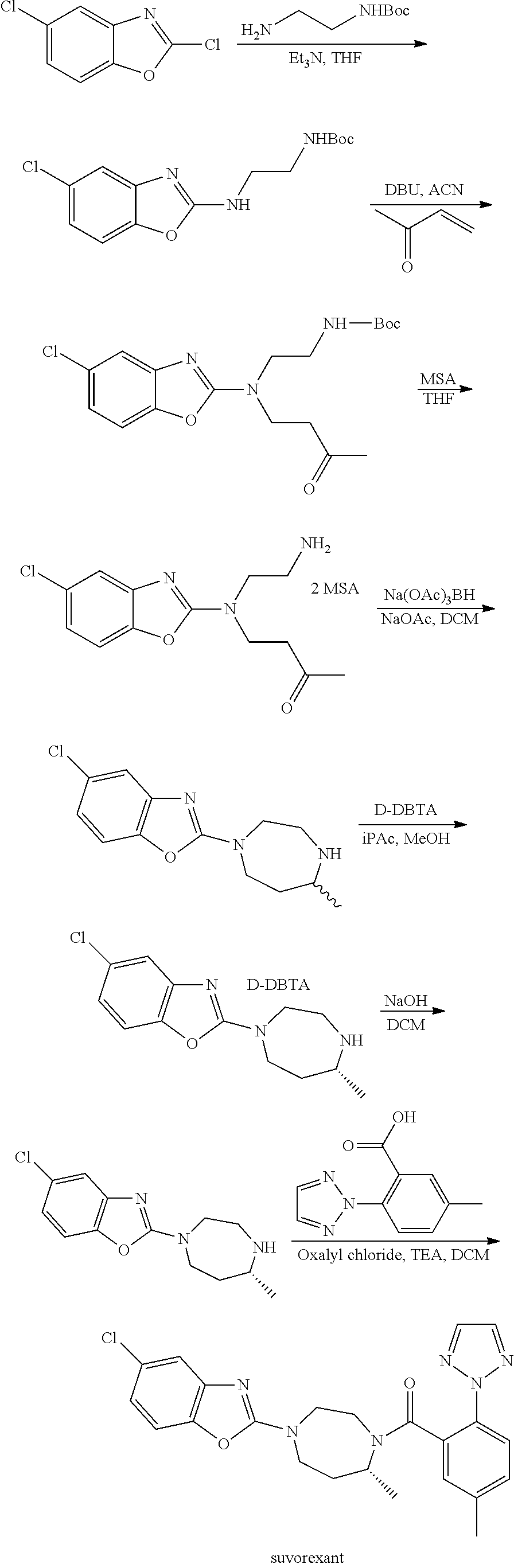
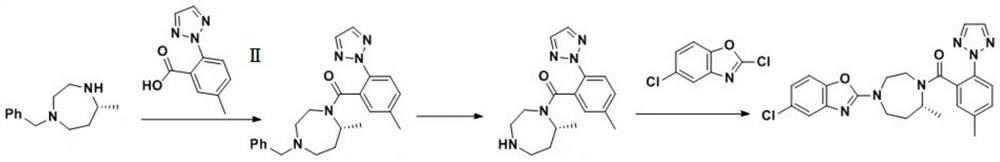
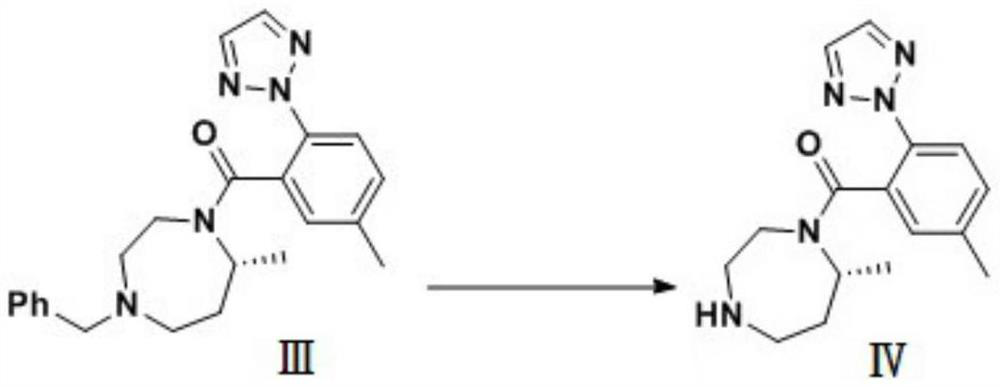
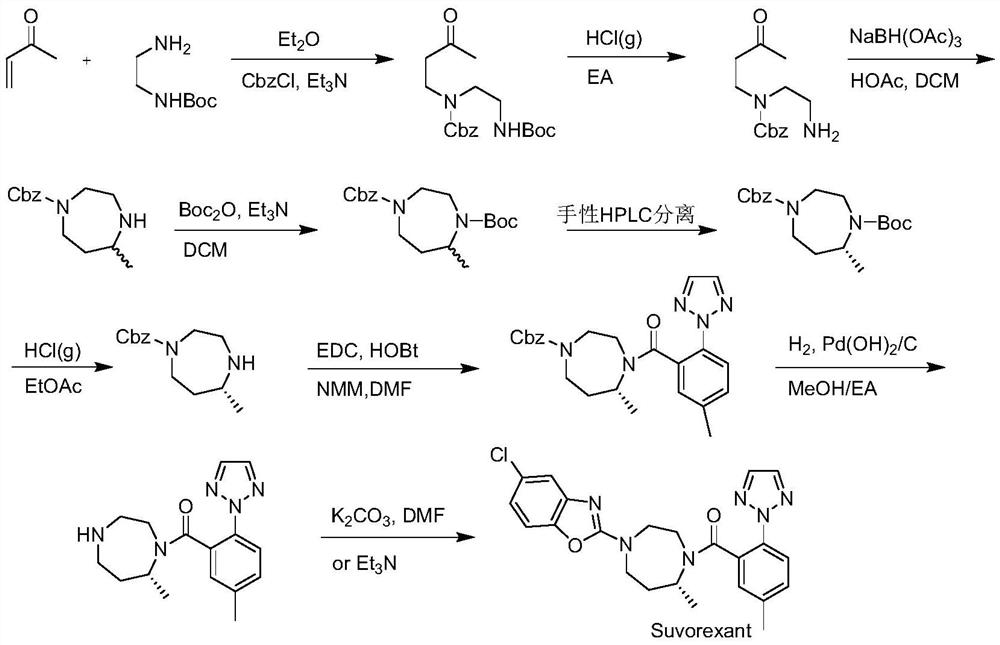
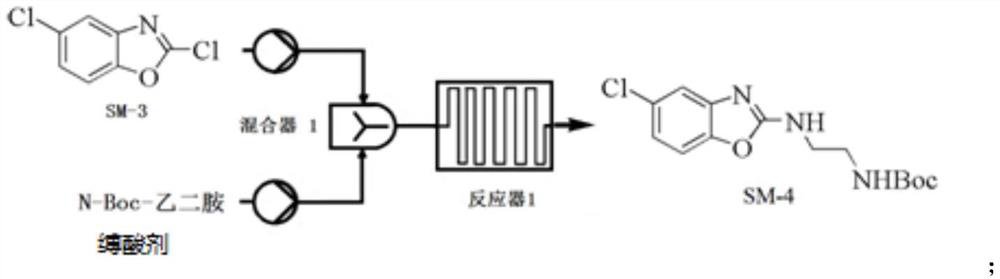
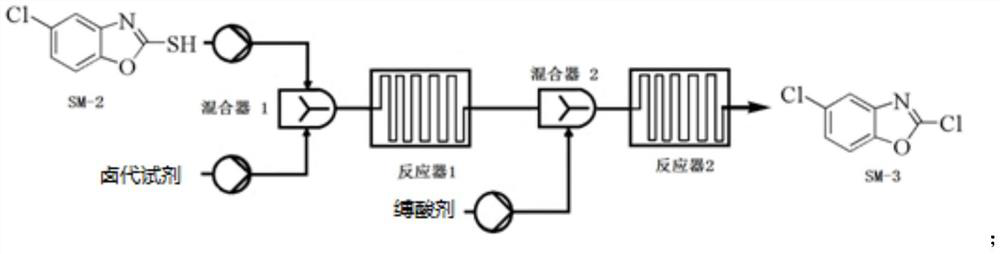
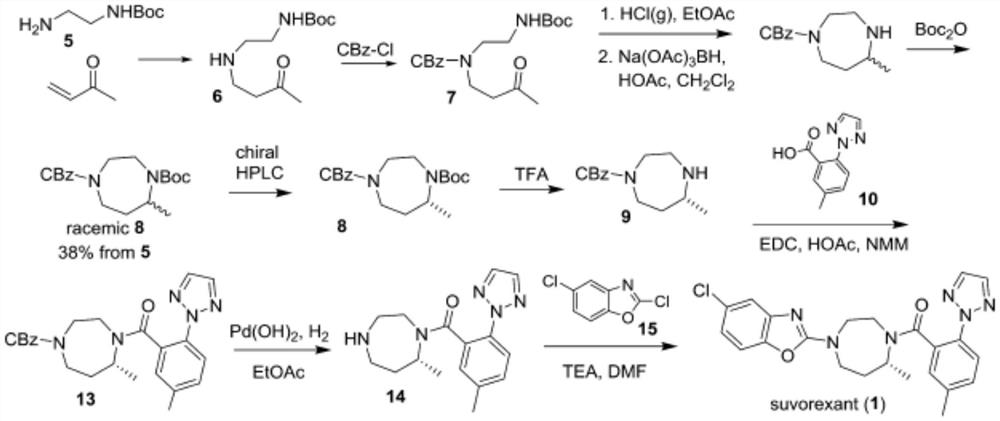
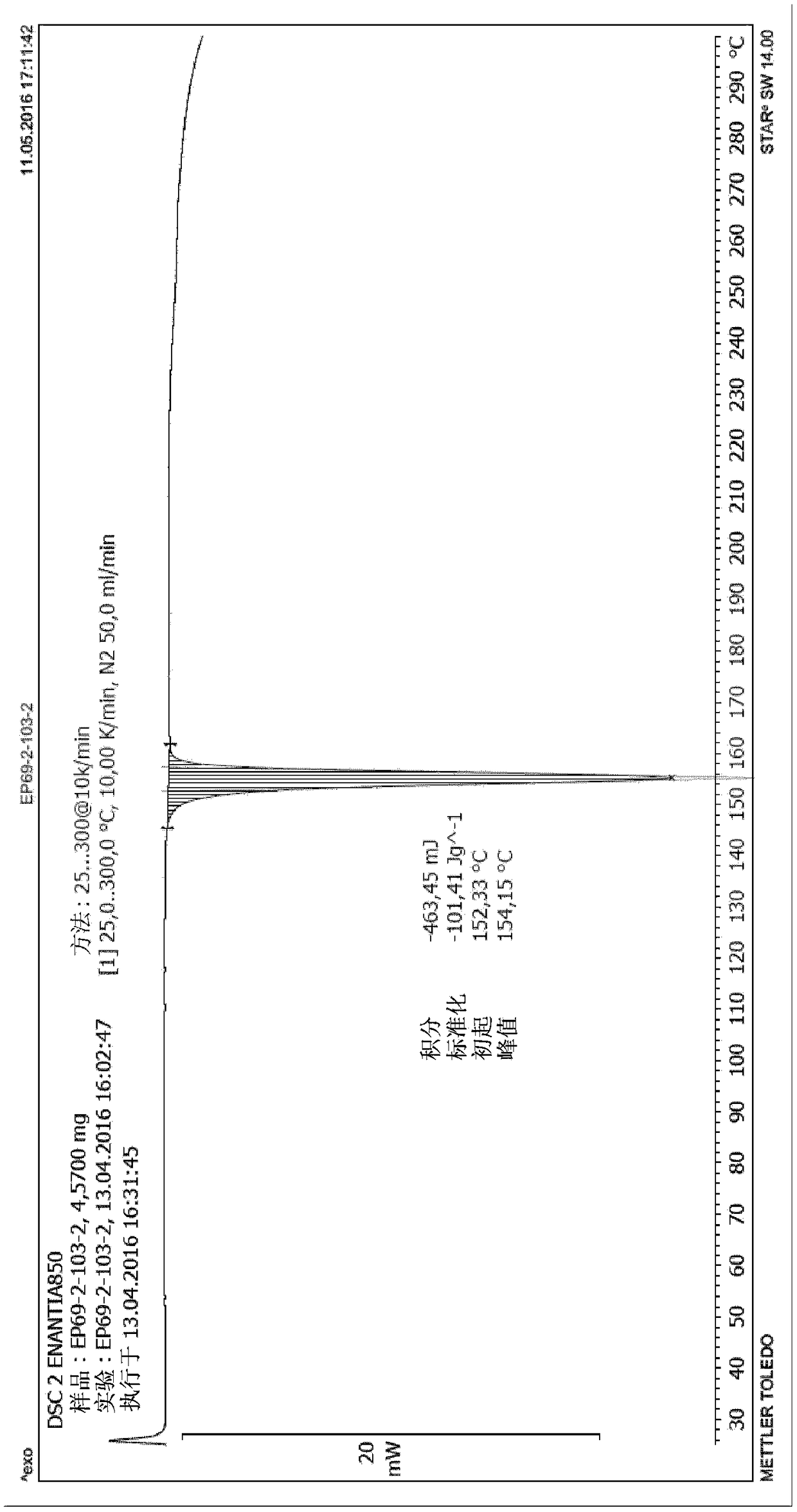
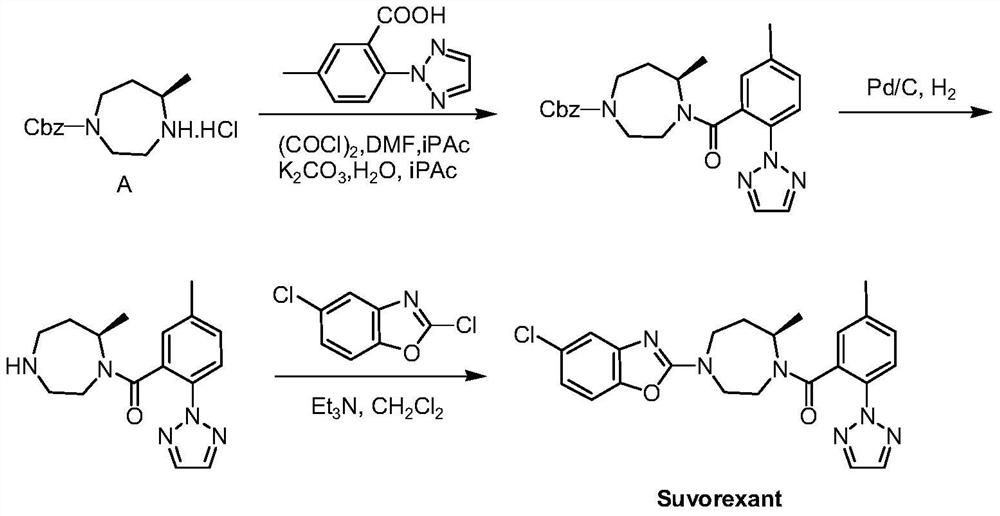
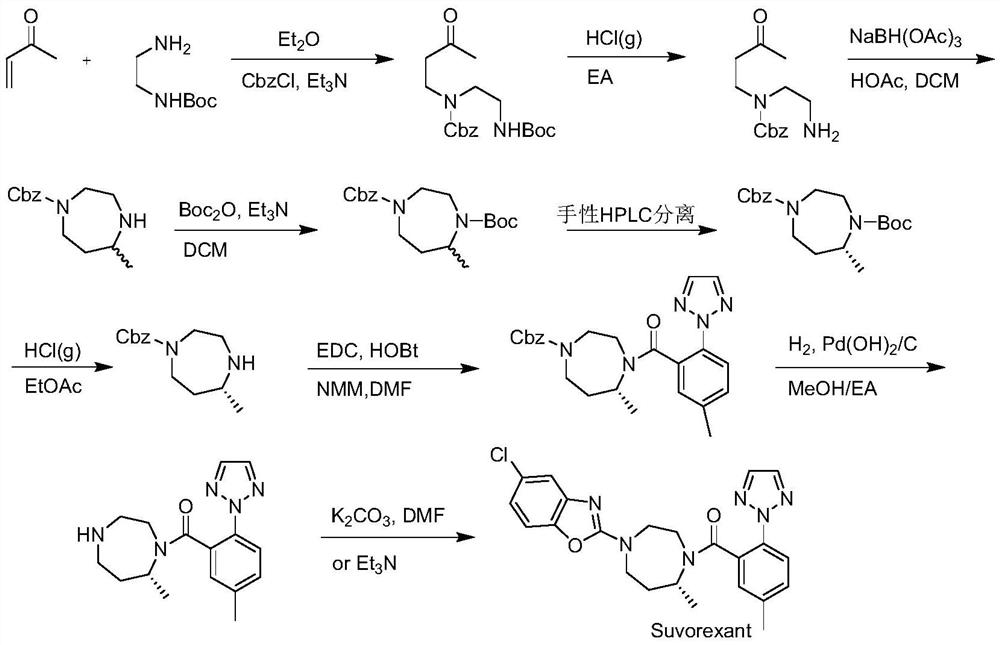
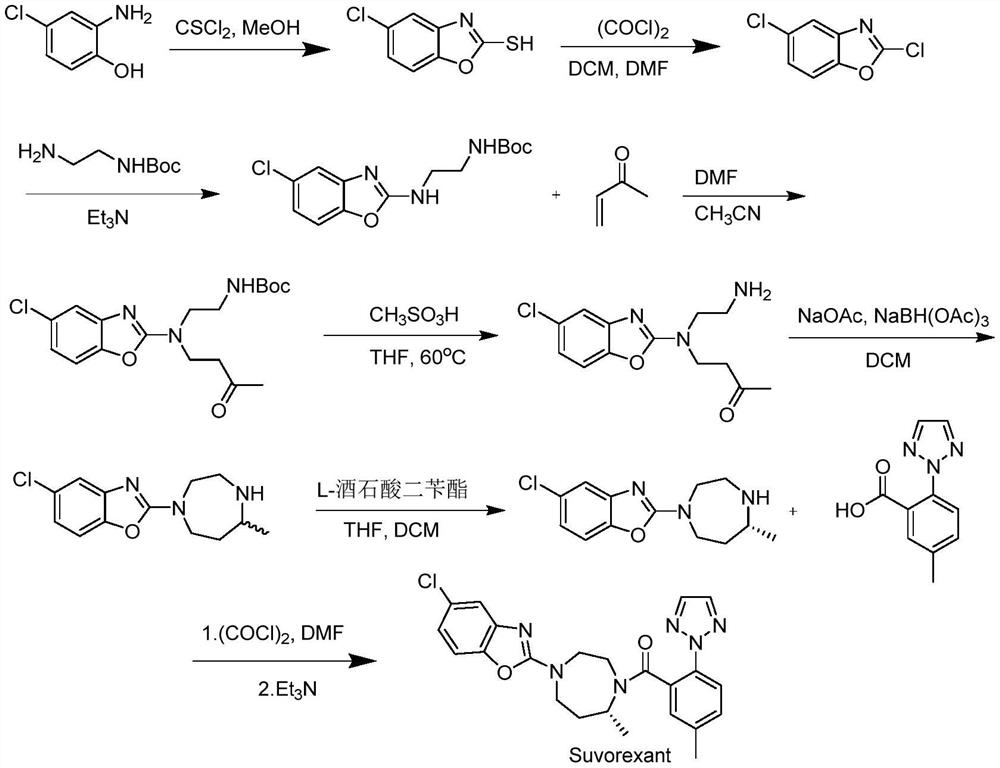
Preparation method of 5-chloro-2-[5-(r)-methyl-1,4-diazepane-1-]benzoxazole
ActiveCN105330657BGuaranteed purityHigh yieldOrganic chemistryBulk chemical productionBenzoxazoleNitrogen
The invention relates to a method for preparing 5-chlorine-2[5-(R)-methyl-1,4-diazacycloheptyl-1-] benzoxazole. The method includes the step of reducing a compound in the formula I (please see the formula in the specification). The invention further relates to a new midbody compound shown in the formula I. 5-chlorine-2[5-(R)-methyl-1,4-diazacycloheptyl-1-] benzoxazole is an important midbody for synthesizing medicine Suvorexant treating sleep disorders. According to the preparation method, initial materials are introduced into a chiral center, in the whole reaction process, reactions and reagents influencing the chiral center are not adopted, the chiral separation or chiral catalystic method which is high in cost and low in yield is avoided, chiral interference reactions do not exist in the technological process, chiral purity of products is guaranteed, only conventional methods and equipment are used, operation is easy, conditions are moderate, lines are short, the yield is high, and the method is suitable for industrial production.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Chiral resolution of an intermediate of suvorexant and cocrystals thereof
InactiveCN109195954ANervous disorderOptically-active compound separationCarboxylic acidPharmaceutical medicine
Relating to processes for preparing suvorexant or its pharmaceutically acceptable salts through the formation of a cocrystal of (R)-benzyl 5-methyl-1,4-diazepane-1-carboxylate hydrochloridewith (R)-(+)-1,1,2-triphenyl-1,2-ethanediol ((R)-TED). This cocrystal provides the resolution of an intermediate of suvorexant, in particular, of(rac)-benzyl5-methyl-1,4-diazepane-1-carboxylateor a hydrochloridesalt thereof. It also relates to a new cocrystal useful in such preparation processes.
Owner:ENANTIA
Preparation method of chiral homopiperazine ring
ActiveCN105367506BGuaranteed purityAvoid pollutionOrganic chemistryBulk chemical productionTreatment sleepPtru catalyst
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Suvorexant intermediate and preparation method thereof
ActiveCN111943945AReduce usageAvoid separationCarbamic acid derivatives preparationOrganic compound preparationEthyl groupMethyl palmoxirate
The invention discloses a suvorexant intermediate and a preparation method thereof. In the suvorexant intermediate, R represents methyl or ethyl, and PG1 and PG2 represent amino protecting groups. Thepreparation method comprises the following steps: carrying out Michael addition reaction to obtain a compound III; removing an amino protecting group from the compound III to generate a compound IV;reducing the compound IV to obtain a compound V; and protecting secondary amine in the compound V by using an amino protecting group to obtain a compound VI. According to the method disclosed by the invention, the use of a highly toxic compound methyl vinyl ketone is avoided, the suvorexant intermediate with the required configuration is obtained by using chiral starting raw materials, the separation by using a chiral resolving agent or chiral HPLC (High Performance Liquid Chromatography) is avoided, and the use of transition metal and a biological enzyme preparation for chiral catalysis is avoided. The reaction conditions have the advantages of simple post-treatment, easiness in separation and purification, high yield, high ee value, easiness in industrialization and the like.
Owner:SHANGHAI INSTITUTE OF TECHNOLOGY
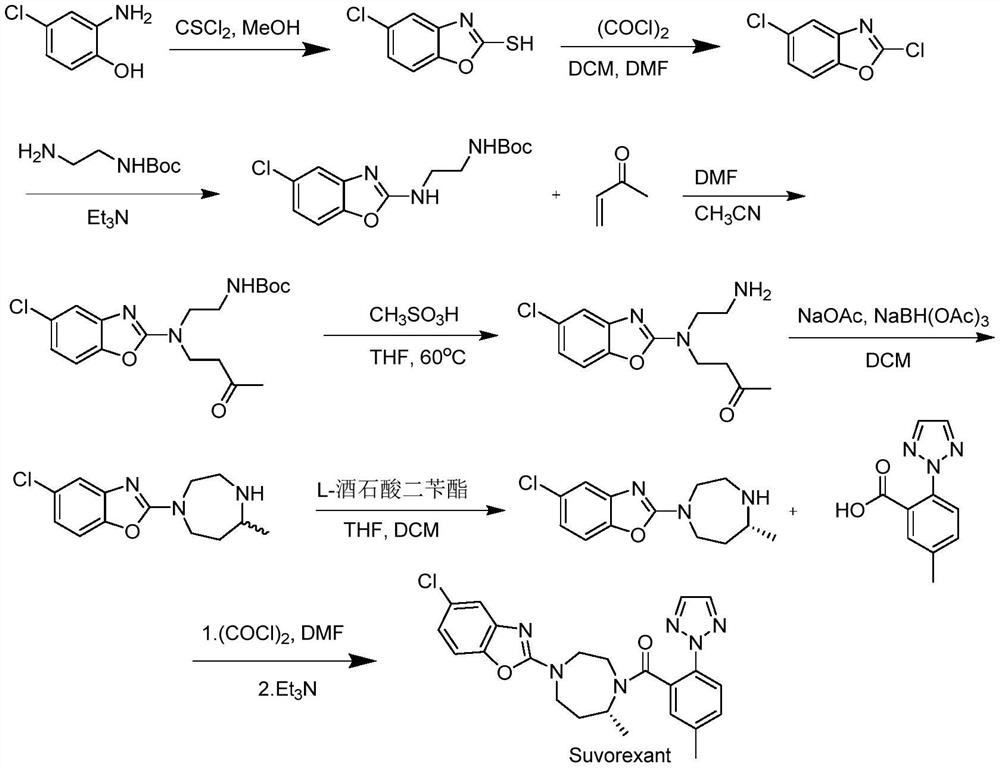
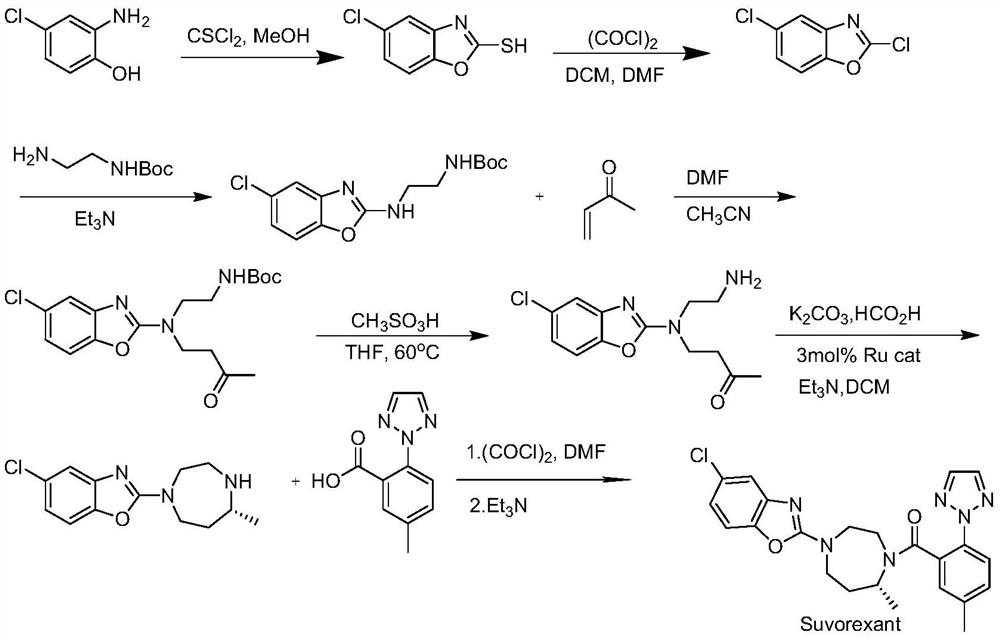
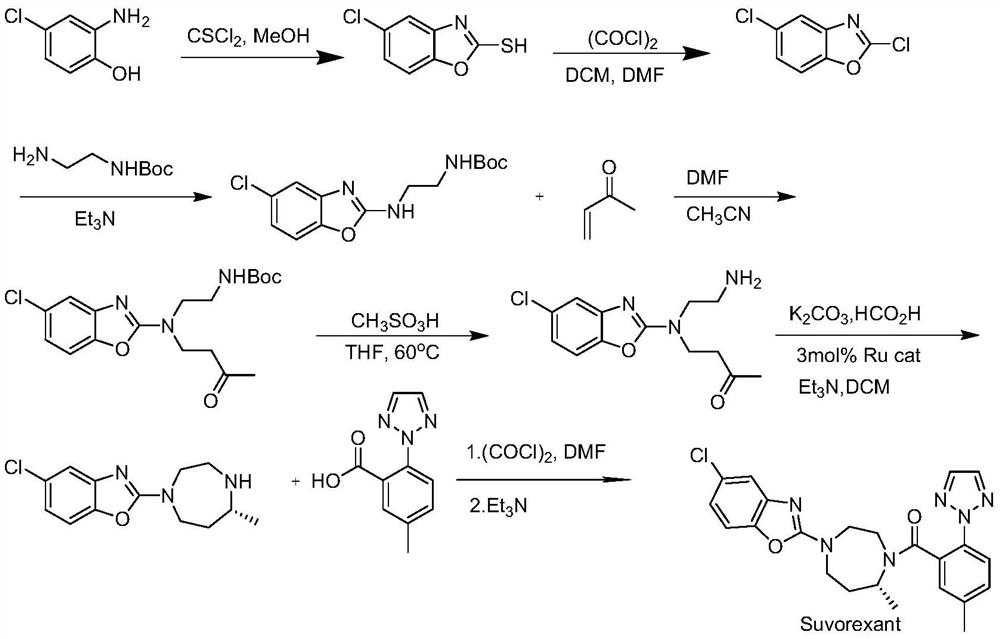
Method for preparing 5-chlorine-2[5-(R)-methyl-1,4-diazacycloheptyl-1-] benzoxazole
ActiveCN105330657AGuaranteed purityHigh yieldOrganic chemistryBulk chemical productionBenzoxazoleNitrogen
The invention relates to a method for preparing 5-chlorine-2[5-(R)-methyl-1,4-diazacycloheptyl-1-] benzoxazole. The method includes the step of reducing a compound in the formula I (please see the formula in the specification). The invention further relates to a new midbody compound shown in the formula I. 5-chlorine-2[5-(R)-methyl-1,4-diazacycloheptyl-1-] benzoxazole is an important midbody for synthesizing medicine Suvorexant treating sleep disorders. According to the preparation method, initial materials are introduced into a chiral center, in the whole reaction process, reactions and reagents influencing the chiral center are not adopted, the chiral separation or chiral catalystic method which is high in cost and low in yield is avoided, chiral interference reactions do not exist in the technological process, chiral purity of products is guaranteed, only conventional methods and equipment are used, operation is easy, conditions are moderate, lines are short, the yield is high, and the method is suitable for industrial production.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Preparation method and application of suvorexant intermediate
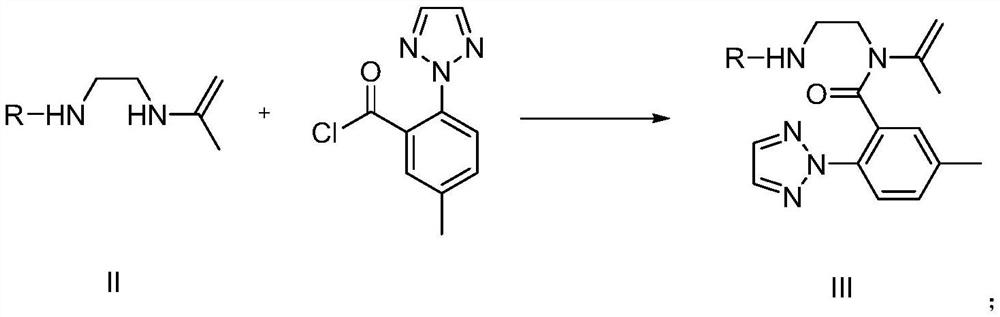
PendingCN112574181AAvoid it happening againMild reaction conditionsOrganic chemistry methodsChemical synthesisToxic gas
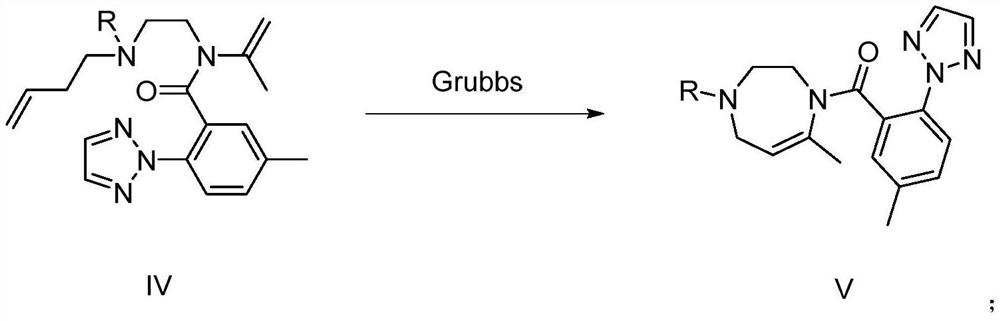
The invention provides a preparation method and application of a suvorexant intermediate, and relates to the technical field of chemical synthesis. The preparation method of the suvorexant intermediate comprises the following step: (e) reacting a compound as shown in a formula IV under the action of a Grubbs catalyst to obtain a compound as shown in a formula V. The Grubbs catalyst (Grubbs second-generation catalyst) is creatively used for construction of the suvorexant intermediate seven-membered nitrogen-containing chiral heterocycle, an inflammable and highly toxic compound methyl vinyl ketone is not needed, inflammable and explosive toxic gas is not needed, reaction is not needed under the conditions of strong acid, strong alkali and high temperature, generation of a large amount of acid liquor and alkali liquor and high-temperature operation can be avoided, the method is mild in reaction condition, environment-friendly, high in safety coefficient and easy to industrialize.
Owner:TIANJIN PHARMA GROUP CORP
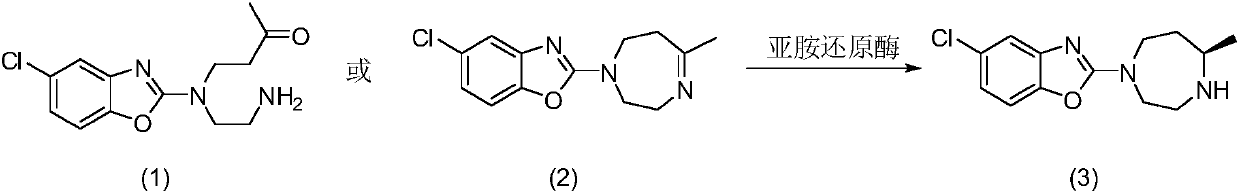
A kind of enzymatic preparation method of Suwo Leisheng key intermediate
The present invention discloses imine reductase StIR (WP_023587323.1) derived from Streptomyces thermolilacinus or imine reductase KcIR (WP_020388085.1) of Kribbella catacumbae or imine reductase SiIR (WP_044567941.1) of Streptomyces iranensis or Leishmania major The imine reductase LmIR of strain Friedlin (CAJ03998.1) or the imine reductase MsIR of Methylocaldum szegediense (WP_026609689.1) or the imine reductase MbIR of Microbulbifer (WP_067084177.1), and use the imine reductase as biological Catalyst to prepare anti-insomnia drug Suvorexan intermediate 5-chloro-2-((R)-5-methyl-[1,4]diazepan-1-yl)benzoxazole. The corresponding imine reductase can catalyze 5-100g / L of the substrate, and the conversion rate is greater than 99%. The method has the remarkable characteristics of mild reaction conditions, no pollution, simple process route, etc., and has great industrial application prospects.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
A kind of preparation method of Suwo Leisheng intermediate
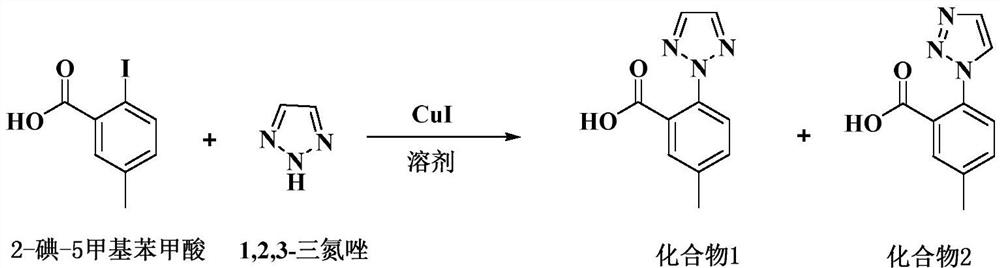
ActiveCN109810067BReduce pollutionLow reaction temperatureOrganic chemistryBulk chemical productionBenzoic acidPtru catalyst
The invention discloses a preparation method of a Suvoryxan intermediate, comprising the following steps: using a trace amount of cuprous iodide as a catalyst, using anhydrous potassium carbonate as a base and acetone as a solvent of three types of solvents, and 2-iodine-5 formazan Ullman reaction between benzoic acid and 1,2,3-triazole, and a high-purity Suvorexan intermediate can be obtained after one ethanol and water mixed solvent purification. The preparation method of the invention fully utilizes the theory of atomic economy, and is easy to operate, low in cost and pollution-free. It is suitable for large-scale industrial production; and after only one refinement, the chromatographic purity can reach more than 99.8%, the isomer is less than 0.1%, and the yield is more than 90%.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Suvorexant key intermediate enzymatic preparation method
The present invention discloses an imine reductase StIR (WP_023587323.1) derived from streptomyces thermolilacinus or an imine reductase KcIR (WP_020388085.1) from kribbella catacumbae or an imine reductase SiIR (WP_044567941.1) from streptomyces iranensis or an imine reductase LmIR (CAJ03998.1) from leishmania major strain friedlin or an imine reductase MsIR (WP_026609689.1) from methylocaldum szegediense or an imine reductase MbIR (WP_067084177.1) from microbulbifer, and the imine reductase is also used as a biological catalyst to prepare anti-insomnia drug suvorexant intermediate 5-chloro-2-((R)-5-methyl-[1,4] diazacyclic heptyl-1-yl)benzoxazole. The corresponding imine reductase can catalyze a substrate of 5-100 g / L and has a conversion rate more than 99%. The method has characteristics of mild reaction conditions, no pollution, simple process routes, etc., and has relatively great industrial application prospects.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Suvorexant tablet and preparation method thereof
PendingCN114344269AIncrease absorption rateGood dissolution effectOrganic active ingredientsNervous disorderLactosePharmaceutical Aids
The invention relates to the technical field of medicines, and provides a suvorexant tablet and a preparation method thereof, and the suvorexant tablet comprises 35-55 parts by weight of a suvorexant solid dispersion, 10-20 parts by weight of lactose, 30-50 parts by weight of microcrystalline cellulose, 11-20 parts by weight of a disintegrating agent and 0.5-3 parts by weight of a lubricant. According to the suvorexant tablet provided by the invention, the suvorexant is subjected to micro-powder treatment, the suvorexant and the copovidone are firstly prepared into the solid dispersion by adopting a hot melting process, and then the solid dispersion and various auxiliary materials are prepared into the tablet, so that the dissolution property of the indissolvable medicine suvorexant is improved, the absorption rate of the suvorexant in a human body is favorably improved, and the curative effect of the suvorexant is improved. Therefore, the bioavailability is improved.
Owner:北京鑫开元医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
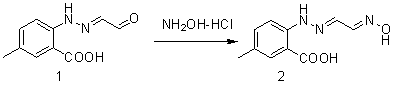
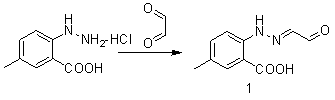


















![Preparation method of 5-chloro-2-[5-(r)-methyl-1,4-diazepane-1-]benzoxazole Preparation method of 5-chloro-2-[5-(r)-methyl-1,4-diazepane-1-]benzoxazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6e0bea86-3dd5-4f23-85e1-a2fe93fab9c0/BDA0000870189010000011.png)
![Preparation method of 5-chloro-2-[5-(r)-methyl-1,4-diazepane-1-]benzoxazole Preparation method of 5-chloro-2-[5-(r)-methyl-1,4-diazepane-1-]benzoxazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6e0bea86-3dd5-4f23-85e1-a2fe93fab9c0/BDA0000870189010000021.png)
![Preparation method of 5-chloro-2-[5-(r)-methyl-1,4-diazepane-1-]benzoxazole Preparation method of 5-chloro-2-[5-(r)-methyl-1,4-diazepane-1-]benzoxazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6e0bea86-3dd5-4f23-85e1-a2fe93fab9c0/BDA0000870189010000022.png)



![Method for preparing 5-chlorine-2[5-(R)-methyl-1,4-diazacycloheptyl-1-] benzoxazole Method for preparing 5-chlorine-2[5-(R)-methyl-1,4-diazacycloheptyl-1-] benzoxazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8049458b-1649-4b14-89f8-42bd6d082097/BDA0000870189010000011.PNG)
![Method for preparing 5-chlorine-2[5-(R)-methyl-1,4-diazacycloheptyl-1-] benzoxazole Method for preparing 5-chlorine-2[5-(R)-methyl-1,4-diazacycloheptyl-1-] benzoxazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8049458b-1649-4b14-89f8-42bd6d082097/BDA0000870189010000021.PNG)
![Method for preparing 5-chlorine-2[5-(R)-methyl-1,4-diazacycloheptyl-1-] benzoxazole Method for preparing 5-chlorine-2[5-(R)-methyl-1,4-diazacycloheptyl-1-] benzoxazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8049458b-1649-4b14-89f8-42bd6d082097/BDA0000870189010000022.PNG)