Continuous flow micro-reaction synthesis method of suvorexant intermediate
A microreactor and reaction technology, applied in organic chemistry and other directions, can solve the problems of high cost and difficult industrial production, and achieve the effects of low cost, simple operation and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
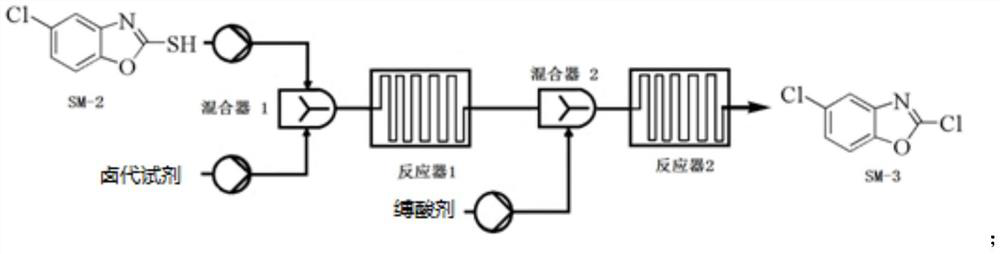
[0107] Example 1 Preparation of Compound SM-2
[0108]
[0109] Compound SM-1 (800 g), EtOH (5L) and ethylthallate (1.79 kg) (1.79 kg) were added to a 10 l 4 flask; 80 ° C for mechanical stirring 4H; TLC was detected, and after the reaction was complete, the reaction liquid was poured down. The pH was adjusted in 8 L of ice water, and the pH was added dropwise to 7, filtered, and the filter cake was washed with water (6L), and the solid was concentrated to concentrate, resulting in a compound SM-2 (1.117 kg), yield 96.7%, purity 95%.
[0110] Compound SM-2 was detected: 1 H NMR (400 MHz, DMSO) δ: 7.31 (m, 2H), 7.53 (D, J = 8.74 Hz, 1H).
[0111] LC-MS: Calculated MW = 185.63g / mol, Found M / Z [M + 1] + = 186.89.
Embodiment 2
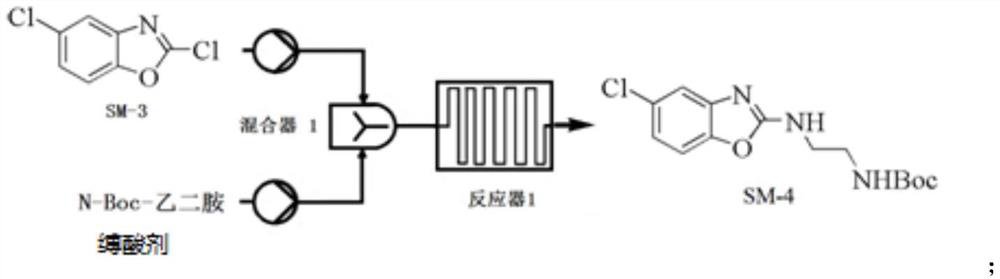
[0112] Example 2 Preparation of Compound SM-3
[0113]
[0114] Precisely weigh the compound SM-2 (1.117 kg), DMF (560 mL) and DCM (11L) thoroughly mixed preparation into uniform liquid spare. The microreactor is disposed and cooled to 5 ° C or less, and the above solution of the compound SM-2, Dichloride SOCL 2 (1120ml) and triethylamine slowly pumped into the microchannel reactor in a set flow (1 ml / min); the reaction liquid flows directly into the 10L NAHCO 3 In the ice aqueous solution, the sedation, the aqueous phase was extracted with DCM (3L), combined with an organic phase, washed once with water (3L), dried over anhydrous sulfate, concentrated under reduced pressure to obtain Compound SM-3 (1.1 kg), yield 97.4%, purity 97%.
[0115] Compound SM-3 was detected: 1 H NMR (400MHz, CDCL 3 δ: 7.67 (D, J = 2.1 Hz, 1H), 7.44 (D, J = 8.8 Hz, 1H), 7.34 (DD, J = 8.8, 2.1 Hz, 1H); 13 C NMR (100MHz, CDCL 3 Δ: 110.9, 12.2, 126.1, 131.0, 142.0, 149.9, 152.34.
[0116] LC-MS: Calcula...
Embodiment 3
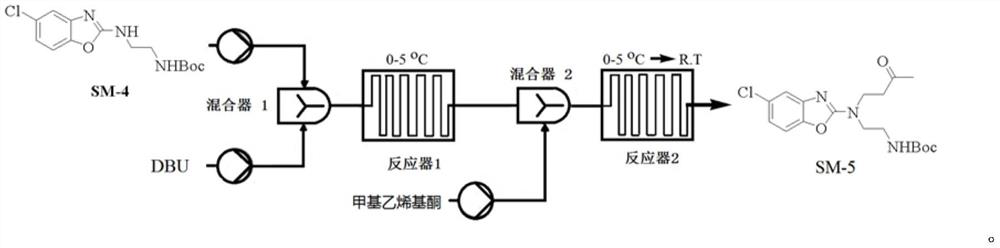
[0117] Example 3 Preparation of Compound SM-4
[0118]
[0119] The solvent was prepared as a solvent in DCM, respectively, a solution of 2 mmol / L compound SM-3 (722 g), 2 mmol / L N-BOC-ethylenediamine (650 g) and 2 mmol / L of triethylamine (586 g), the reaction temperature was maintained at Between 5 ° C -10 ° C, three raw materials were pumped into the microreactor system in a set flow (1 ml / min), and the TLC showed that the feedstock was completed; the reaction liquid was directly injected into 15L ice water, and the mixture was allowed to stand after 2min The separation solution, the aqueous phase was extracted once with DCM (3L), combined with the organic phase, washed once with water (5L), dried over anhydrous sulfate, concentrated under reduced pressure to obtain crude, crude EA: PE (400 ml: 2000ml) Ged (4h ), Filtration, filter cake was washed with petroleum ether (1 L), and the concentrated filter cake was concentrated to give compound SM-4 (850 g), yield 71.3%, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com