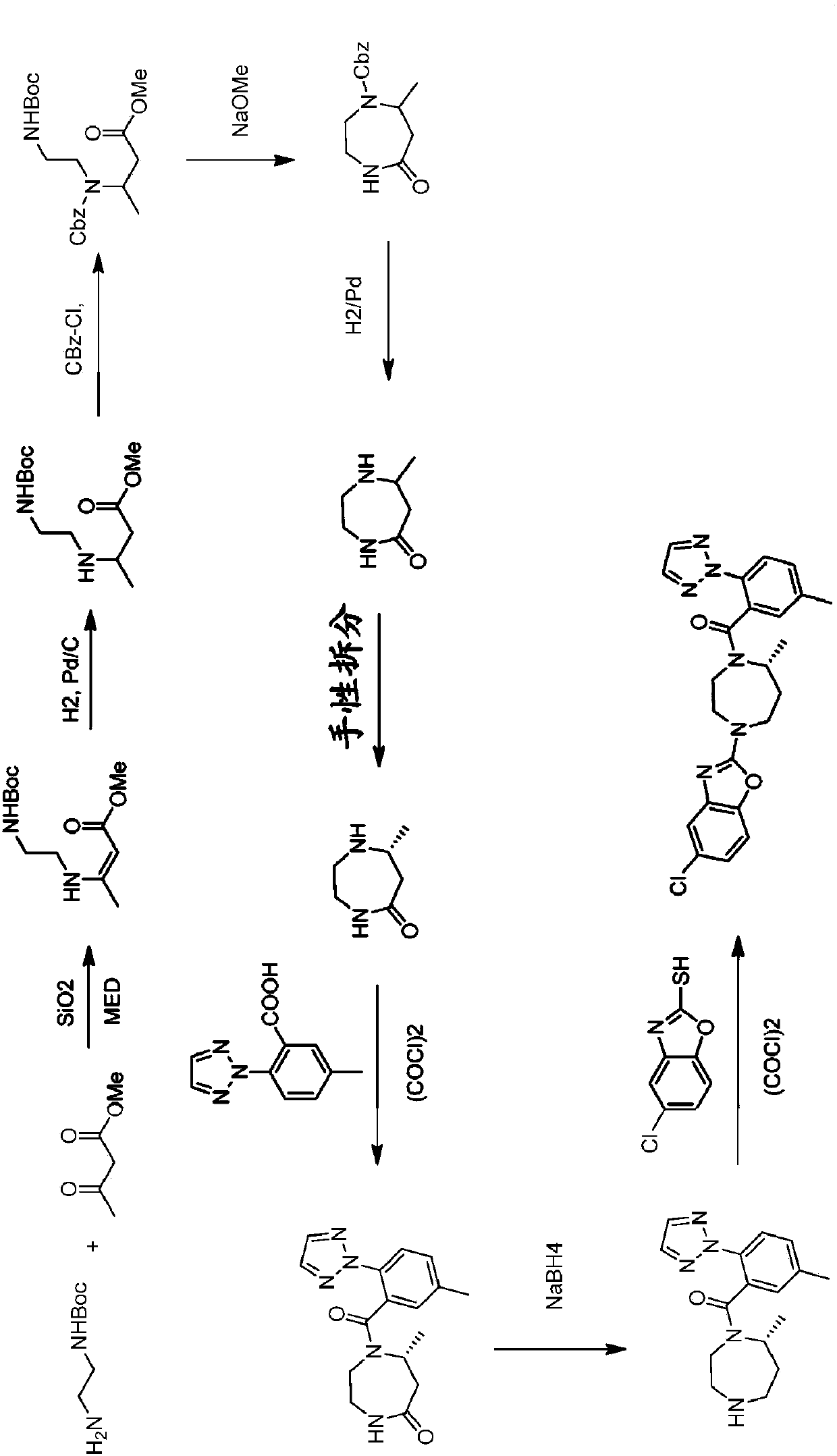
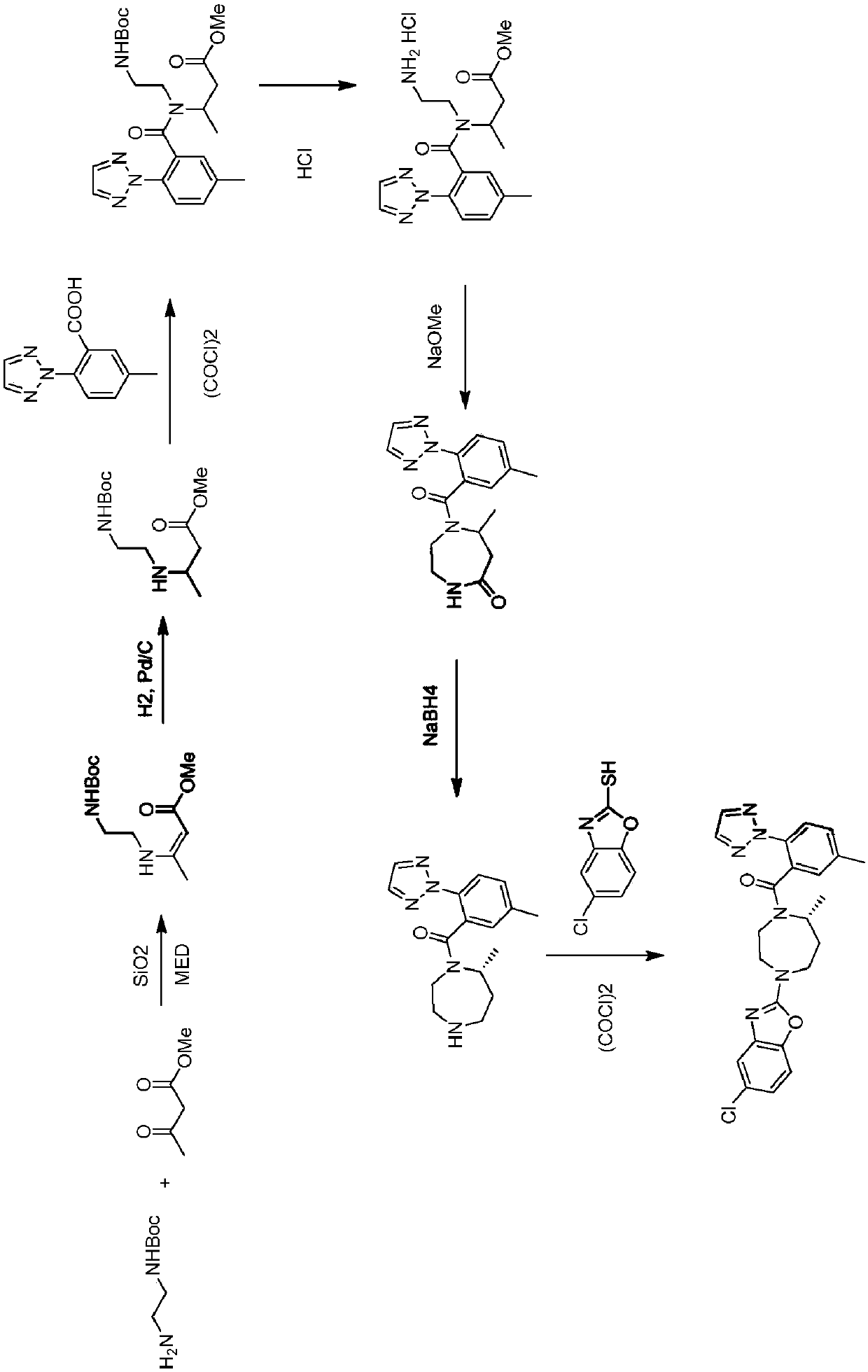
Novel routes of synthesis for preparation of suvorexant
一种化合物、组合物的技术,应用在制备苏沃雷生的新合成途径领域,能够解决不适合制备规模等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0698]
[0699] In the above table, PG 1 The preferred choice is Boc, PG 2 The preferred choice is Cbz.
[0700] Such as R 1 With R 1a Different, or like R 2 With R 2a Different, or like R 1a And R 2a Respectively with R 1a And R 2a Different, perform step (a4). According to the corresponding group to be replaced, step (a4) includes one or more steps, such deprotection step and / or protection step and / or coupling step with the compound of the following structural formula
[0701]
[0702] And / or the coupling step with the compound of the following structural formula
[0703]
[0704] The compound of structural formula (II) is obtained.
[0705] According to the preferred embodiment, step (a) of the present invention includes, for example:
[0706] (a1) Reaction to form a compound of structural formula (III)
[0707]
[0708] Add the compound of structural formula (IV)
[0709]
[0710] Get the compound of structural formula (V)
[0711]
[0712] Where: R 1a For PG 1 , Where: R 2a For H, PG...
Embodiment 1
[1340] Example 1: Preparation of 3-((2-((tert-butoxycarbonyl)amino)ethyl)amino)butan-2-acid (Z)-methyl ester
[1341]
[1342] Take Boc-ethylenediamine (84.3g, 500mmol) and dissolve it in CH 2 Cl 2 (110ml), transfer to 500ml Schmizo, and cool to 10°C. Add silica gel (120g) in portions and use CH 2 Cl 2 (50ml) Dilute the slurry. Methyl acetoacetate (54ml, 500mmol) was added, the reaction mixture was stirred at 20°C, and the progress of the reaction was monitored by GC. After one hour, the reaction was judged to be complete. Filter out the silica gel, use CH 2 Cl 2 (250ml) Wash the filter cake. The pale yellow solution was concentrated under reduced pressure to obtain enamine (127.9 g) as a pale yellow oil.
[1343] 1 H NMR(300MHz, CDCl 3 ): δ=8.57(br s, 1H), 4.84(br s, 1H), 4.47(s, 1H), 3.61(s, 3H), 3.33(m, 2H), 3.23(m, 2H), 1.91( s, 3H), 1.43 (s, 9H). 13 C NMR(75MHz, CDCl 3 ): □=170.9, 162.0, 155.9, 82.7, 79.6, 50.0, 42.8, 41.3, 28.3, 19.3. All data are consistent with the data...
Embodiment 2
[1345] Preparation of methyl 3-((2-((tert-butoxycarbonyl)amino)ethyl)amino)butyrate by hydrogenation
[1346]
[1347] In the presence of 73 g Pd / C, a solution of enamine (58.7 g, 227 mmol) in MeOH (650 ml) was hydrogenated at 55° C. and a pressure of 3 bar. The reaction was monitored by GC. After complete conversion (about 7 hours), the suspension was filtered through a K150 filter and the solid was washed with MeOH. The solution was concentrated under reduced pressure, dissolved in MeOH (300 ml), and distilled under reduced pressure to obtain β-amino ester (51.6 g, 87%) as an oil.
[1348] 1 H NMR(300MHz, CDCl 3 ): δ = 5.28 (br s, 1H, NH), 3.99 (br s, 1H, NH) 3.66 (s, 3H, OCH3), 3.13-3.25 (overlapping m, 3H, CH 2 +CH), 2.78(m, 2H, CH 2 ), 2.48(m, 2H, CH 2 ), 1.41(s, 9H, C(CH 3 ) 3 ), 1.15(d, J=6.3Hz, 3H, CH 3 ). 13 C NMR(75MHz, CDCl 3 ): δ=172.2, 156.1, 79.2, 51.6, 50.1, 45.9, 40.6, 39.8, 28.3, 19.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com