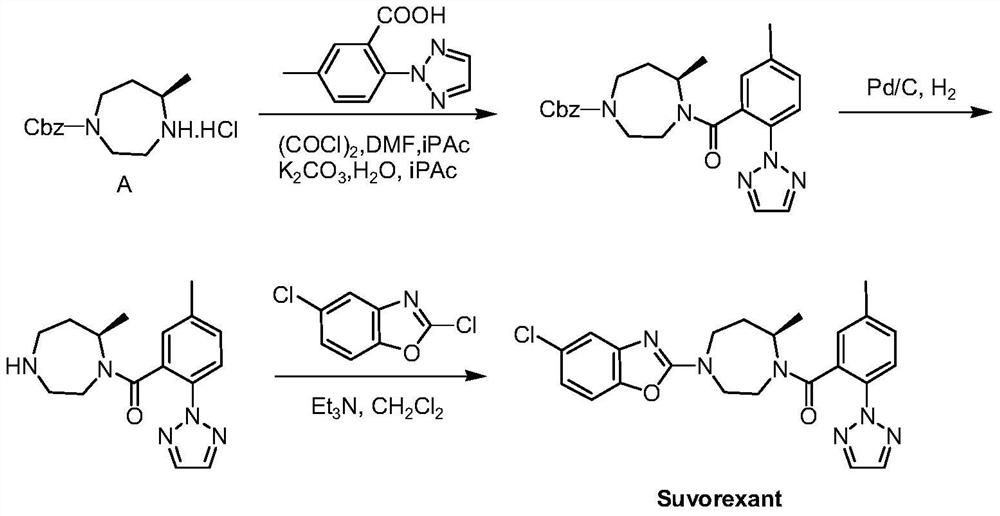
Preparation method of chiral homopiperazine ring
A kind of ring-closure reaction, compound technology, applied in the intermediate compound shown in formula Int, in the field of preparation of chiral homopiperazine ring, can solve problems such as being unsuitable for industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] route one
[0123]
Embodiment 1-1
[0124] Example 1-1: Synthesis of N-benzyl-N-(3-(R)-tert-butoxycarbonylaminobutanyl)-2-chloroacetamide (compound 2)
[0125]
[0126] Chloroacetyl chloride (12.5 g, 0.11 mol) was slowly added to 80 mL of dichloromethane solution to obtain a dichloromethane solution of chloroacetyl chloride. 3-(R)-tert-butoxycarbonylamino-1-N-benzylbutylamine (27.8g, 0.1mol) represented by formula SM-1 was dissolved in 200mL of dichloromethane, triethylamine (12.2 g, 0.12mol), cooled to -5~0°C, slowly added dropwise the dichloromethane solution of chloroacetyl chloride, the temperature in the system was controlled not to exceed 10°C during the dropwise addition, after the dropwise addition was completed, the temperature was raised to room temperature, and stirred for another 2 hours , TLC monitors the reaction, after the reaction finishes, 50 milliliters of ice water is added in the reaction solution, separates the organic phase, extracts the aqueous phase with 100 milliliters of dichlorometh...
Embodiment 1-2
[0127] Example 1-2: Synthesis of 1-benzyl-4-tert-butoxycarbonyl-5-(R)-methyl-1,4-diazepan-2-one (compound 3)
[0128]
[0129] Compound 2 (32g, 0.09mol) was dissolved in anhydrous N,N-dimethylformamide (DMF, 350mL). After it was completely dissolved, anhydrous cesium carbonate (58.5g, 0.18mol) was added, and the temperature was slowly raised to 75°C, maintain the reaction for 16 hours. After the reaction, add 500 ml of water to the reaction solution, extract with ethyl acetate, combine the extracts, wash with saturated sodium chloride solution, dry the organic phase with anhydrous sodium sulfate, and concentrate , recrystallized from a mixture of ethyl acetate and n-heptane (volume ratio 1:10) to obtain compound 3 as a waxy off-white low-melting solid weighing 26.6 g with a yield of 93%. 1 H NMR (400MHz, CD3OD): δ=7.31(m,5H),4.04(s,2H),3.90(m,1H),3.70(s,2H),2.95(m,1H),2.82(m,1H ), 1.97(m, 1H), 1.63(m, 1H), 1.42(s, 9H), 1.12(d, J=7Hz, 3H).LC-MS(ESI) m / z 319.31([M+H] + ). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com