Suvorexant key intermediate enzymatic preparation method
A technique of asana and reductase, which is applied in the field of medicine, can solve the problems of unfavorable separation and purification, by-products, and the theoretical yield is only 50%.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the acquisition of highly expressed genetically engineered bacteria
[0024] The whole gene synthesis was completed by Shanghai Xuguan Company.
[0025] According to the gene StIR (WP_023587323.1) of Streptomyces thermolilacinus, the gene KcIR (WP_020388085.1) of Kribbellacatacumbae, the gene SiIR (WP_044567941.1) of Streptomyces iranensis, the gene LmIR (local1039zeet98) of Leishmania major strain Friedlin, the gene Mdiumset98 MsIR (WP_026609689.1) and Microbulbifer gene MbIR (WP_067084177.1) were respectively codon-optimized in order to enable the gene to be expressed in the E. coli expression host. And add corresponding enzyme cutting sites at both ends of the gene, and construct them into corresponding vectors to obtain genetically engineered bacteria IR1, IR2, IR3, IR4, IR5, and IR6.
[0026] Transform the prepared recombinant vector into Escherichia coli BL21, Rosetta or Origami by conventional methods to construct a genetically engineered bacterium...
Embodiment 2
[0027] The cultivation of embodiment 2 genetically engineered bacteria and the preparation of resting cells
[0028] Pick a single colony on the plate and inoculate it into 5ml of fermentation medium containing corresponding antibiotics, cultivate it for about 15 hours as a seed solution, inoculate it into 600ml of fermentation medium according to the inoculation amount of 1%, and cultivate it on a shaker at 37°C and 200rpm to OD 600 = about 0.6-0.8, add IPTG with a final concentration of 0.1 mM to induce for more than 10 h, and collect the bacteria by centrifuging the culture solution at 8000 rpm.
Embodiment 3
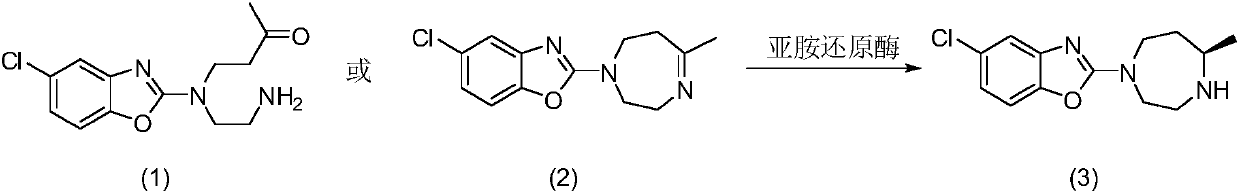
[0029] Example 3 Asymmetric reductive amination of mesylate of formula (1) catalyzed by resting cells of IR1
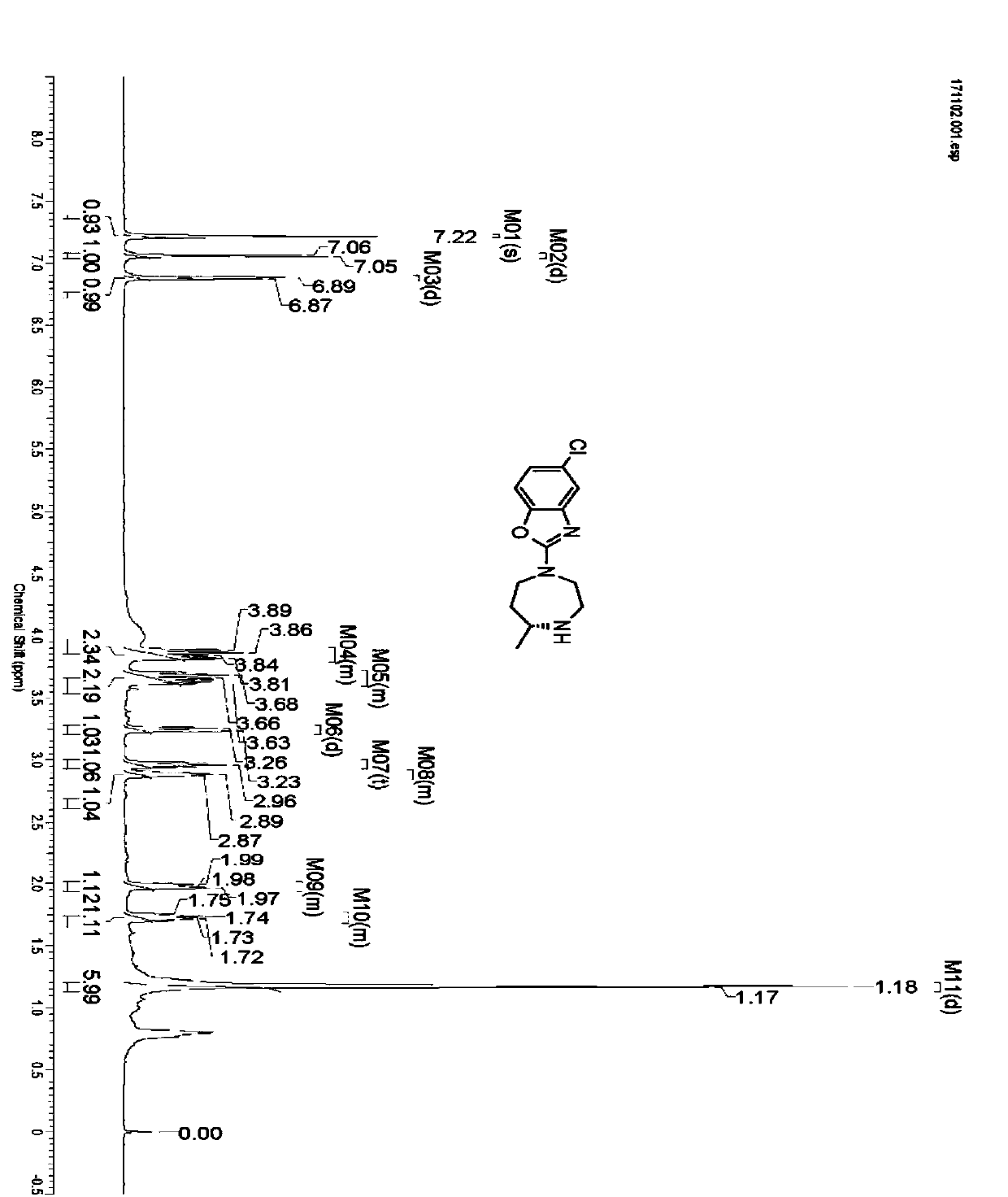
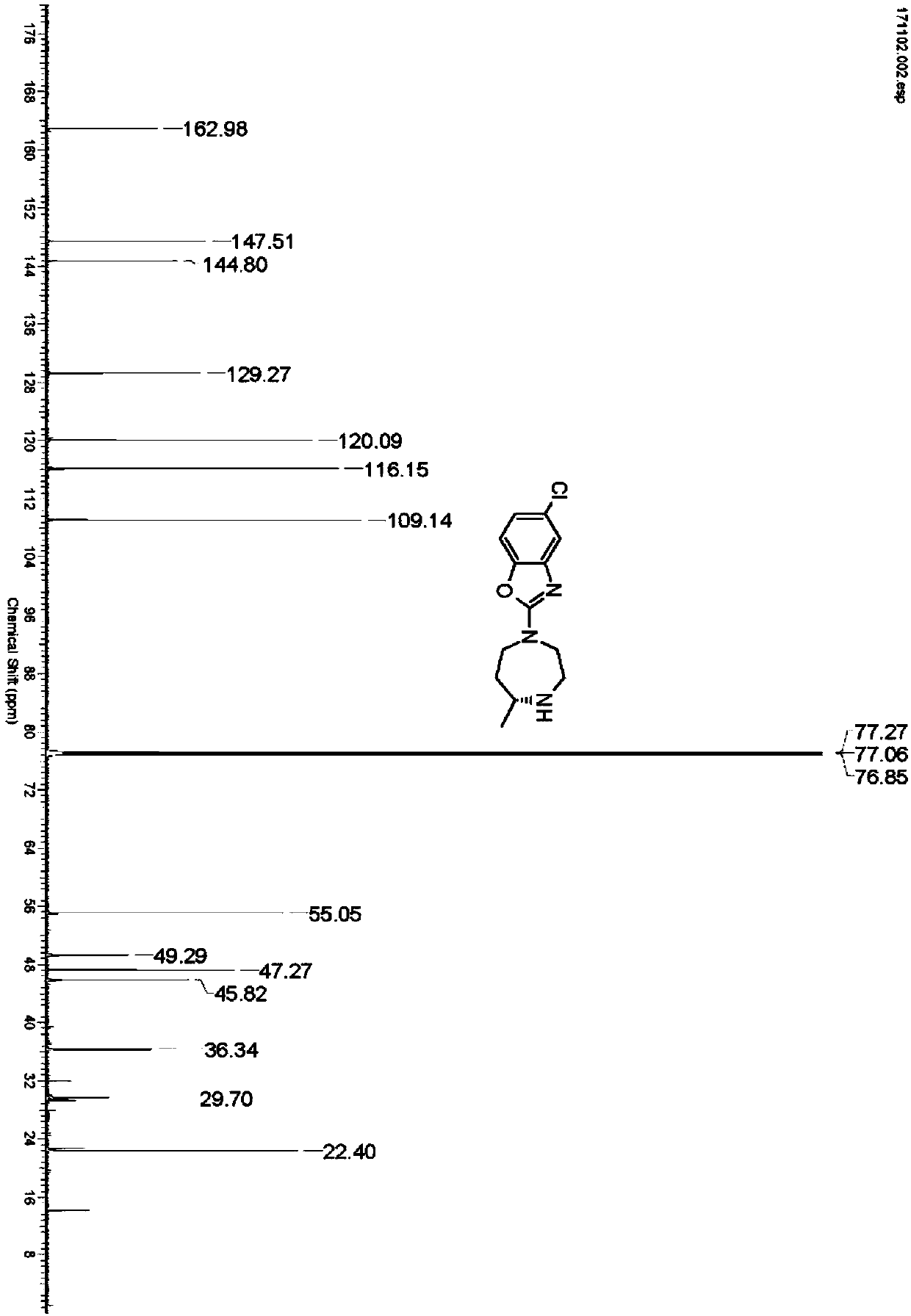
[0030] Take 2.5g of IR1 resting cells and resuspend in 100mL sodium phosphate buffer (100mM, pH 7.2), add glucose (1 g), NADP + (5mg), GDH enzyme powder (50mg), 4-[(2-aminoethyl)(5-chloro-2-benzoxazolyl)amino]-2-butanone-bis(methanesulfonate)( 0.5g), use 10% sodium carbonate solution to control pH 7.0, react at 30°C for 24 hours, thin-layer chromatography detection shows that the reaction is complete, adjust pH to 9.0 with 10% sodium carbonate solution, extract with ethyl acetate (100mL*3) , dried over anhydrous sodium sulfate and spin-dried, separated and purified by column chromatography to obtain 5-chloro-2-((R)-5-methyl-[1,4]diazepan-1-yl)benzo Oxazole 0.252g, yield 90%, ee value>96%. 1H NMR (600MHz, CDCl 3 ):δ7.22(s,1H),7.06(d,J=8.4Hz,1H),6.88(d,J=8.4Hz,1H),3.79-3.91(m,2H),3.59-3.72(m ,2H),3.25(d,J=13.9Hz,1H), 2.96(t,J=11.4Hz,1H),2.84-2.92(m,1H),1.94-2.02(m,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com