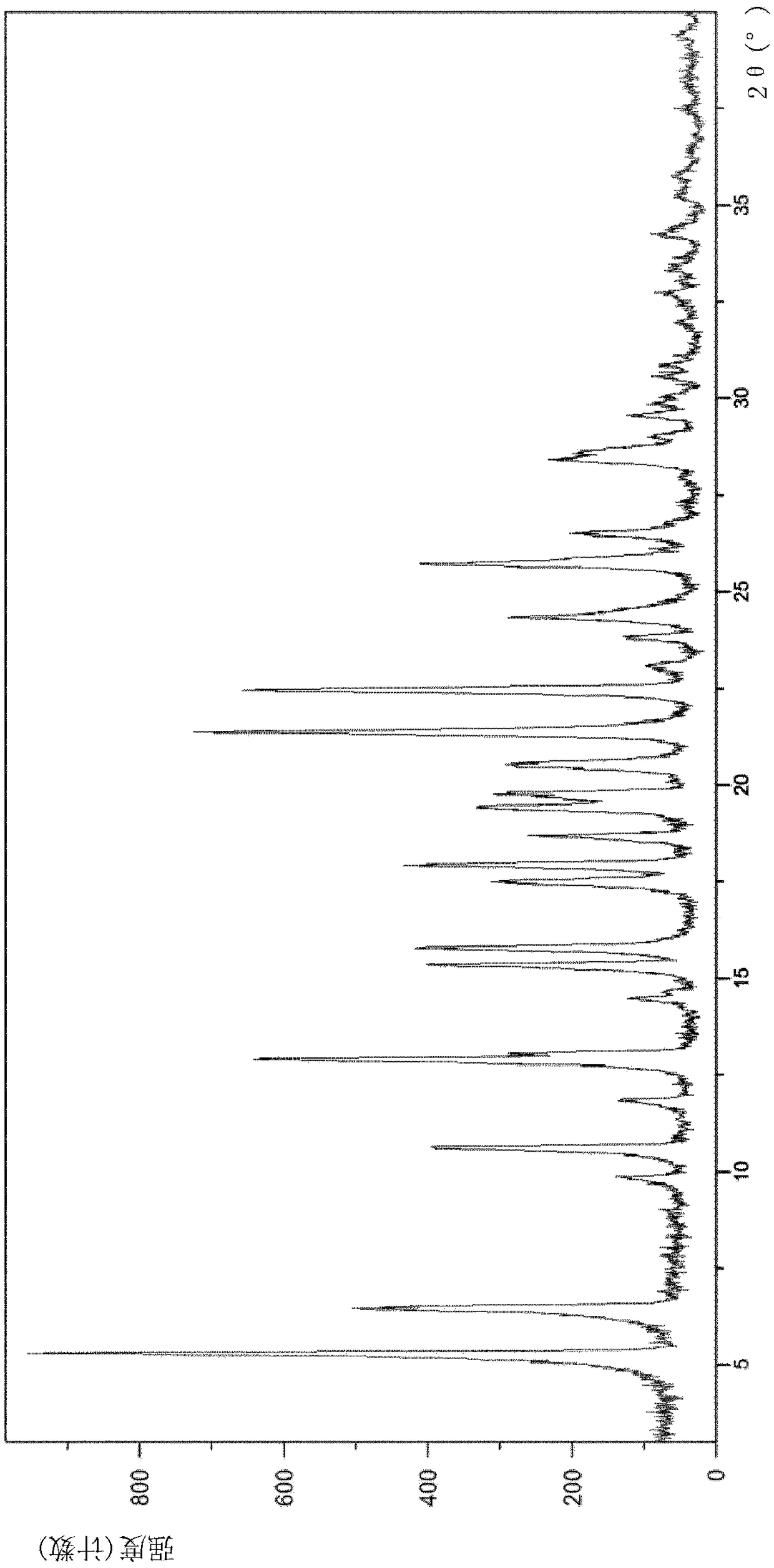
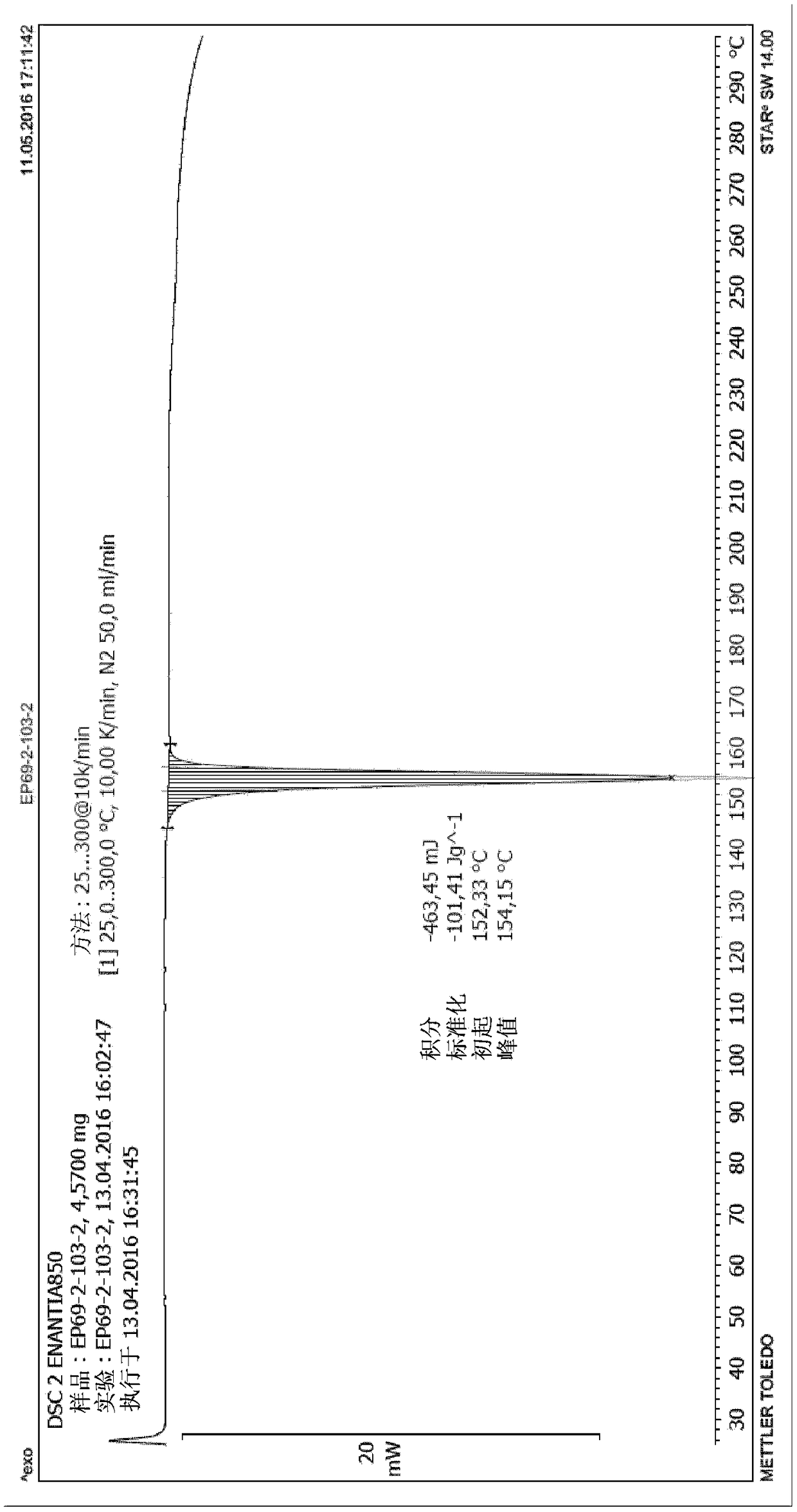
Chiral resolution of an intermediate of suvorexant and cocrystals thereof
A technology for producing formulas and derivatives, which can be used in the separation of optically active compounds, organic racemization, nervous system diseases, etc., and can solve problems such as low yield, large amounts of waste, and low enantiomeric excess
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0097] In a specific embodiment of the method for preparing suvorexan disclosed above, X in compound (IV) is chlorine. In another specific embodiment of the method for preparing Suvorexan, Y in compound (VII) is bromine or chlorine.
[0098] Deprotection of the Cbz group can be achieved, for example, by using H 2 , Pd(OH) 2 , EtOAc hydrogenolysis. The amino protecting group can be introduced and removed by other procedures known in the art (see T.W. Greene and G.M. Wuts, Protective Groups in Organic Synthesis, Third Edition, Wiley, N.Y., 1999, pp. 531-535).
[0099]The group PG is a suitable orthogonal amino protecting group. As used herein, the term "suitable orthogonal amino-protecting group" means to cover any amino-protecting group other than the protecting group Cbz to protect other amino groups of compounds of formula (R)-(III), which are sensitive to the selected Cbz cleavage conditions ( such as hydrogenolysis) are stable. Representative protecting groups for amin...
Embodiment 1
[0106] Example 1: Detection of (R)-5-methyl-1,4-diazepane-1-carboxylate benzyl ester hydrochloride and (R)-(+)-1,1,2-tri Phenyl-1,2-ethylene glycol cocrystal form A and (S)-5-methyl-1,4-diazepane-1-carboxylate benzyl ester hydrochloride with (S)-( -)-1,1,2-Triphenyl-1,2-ethanediol co-crystal
[0107] The raw materials used were (rac)-5-methyl-1,4-diazepane-1-carboxylate benzyl ester HCl and (R)-TED (1:1) or (S)-TED ( 1:1).
[0108] (S)-5-Methyl-1,4-diazepane-1-carboxylate benzyl ester hydrochloride and (S)-(-)-1,1,2-triphenyl-1, Co-crystals of 2-ethanediol were obtained by slurrying and wet milling in acetonitrile (ACN).
[0109] (S)-5-Methyl-1,4-diazepane-1-carboxylate benzyl ester hydrochloride and (S)-(-)-1,1,2-triphenyl-1, Co-crystals of 2-ethylene glycol were also slurried by using isopropanol, ethyl acetate, acetone and toluene as solvents and using isopropanol, ethyl acetate, acetone, tetrahydrofuran, tert-butyl methyl ether, dichloromethane and Obtained by wet gri...
Embodiment 2
[0112] Example 2: Preparation of (S)-5-methyl-1,4-diazepane-1-carboxylic acid benzyl ester hydrochloride and (S)-(- )-1,1,2-Triphenyl-1,2-ethanediol co-crystal and (R)-5-methyl-1,4-diazepane-1-carboxylic acid benzyl ester salt Cocrystal A of acid salt and (R)-(+)-1,1,2-triphenyl-1,2-ethanediol
[0113] In an Eppendorf tube, racemic 5-methyl-1,4-diazepane-1-carboxylic acid benzyl ester hydrochloride (19.8 mg, 0.07 mmol) and (S)-1,1, 2-Triphenyl-1,2-ethanediol (20.1 mg, 0.07 mmol) was suspended in acetonitrile (0.2 mL). The resulting suspension was stirred at room temperature for 15 hours (overnight). Then, the solid was recovered by centrifugation and dried under high vacuum at room temperature.
[0114] Can be obtained by the same method starting from (R)-1,1,2-triphenyl-1,2-ethanediol instead of (S)-1,1,2-triphenyl-1,2-ethanediol (R)-5-Methyl-1,4-diazepane-1-carboxylate benzyl ester hydrochloride and (R)-(+)-1,1,2-triphenyl-1, 2-Ethylene glycol type A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com