Preparation method of raw drug suvorexant
A technology for raw materials and intermediates, which is applied in the field of preparation of raw materials Suvorexan, can solve the problems of high reaction temperature, unfavorable guarantee of yield, and amides affecting affinity, etc., and achieves the effect of simple separation operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
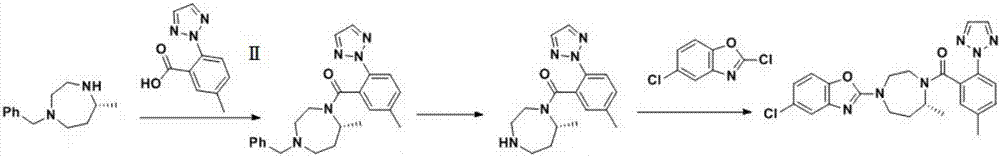
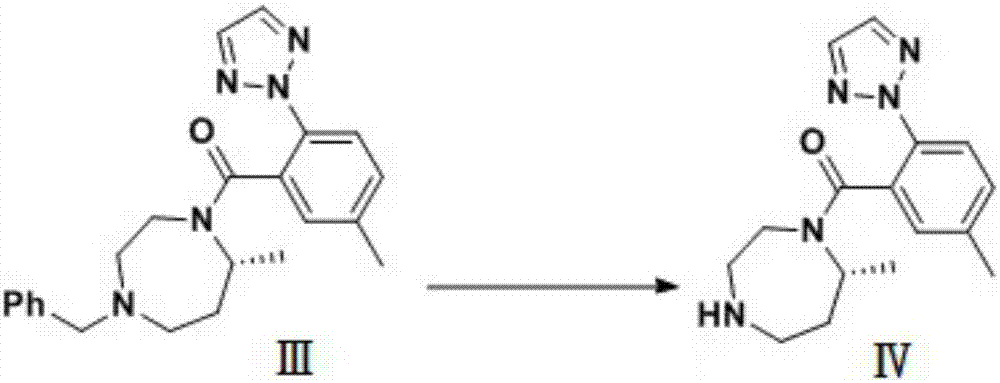
[0038] Embodiment 1 adopts as the synthetic route of background technology, and wherein S1 technological process is:
[0039] Add 48 kg (235 mol) of intermediate I to the reaction kettle, add 300 ml of dichloromethane, start stirring, add 48 kg (235 mol) of intermediate II; after adding alkali and stirring well, add 45 kg of condensing agent EDC.HCL, and control the temperature at 25°C when adding Next, keep warm at 24-27° C. for reaction, monitor by TLC, TLC shows that the reaction is complete, and post-processing to obtain 87 kg (223 mol) of intermediate III, with a yield of 94.9%.
Embodiment 2
[0041] Example 2 uses dimethylformamide as the reaction solvent, uses pyridine as the base, and uses N,N'-carbonyldiimidazole and tris(2,6-dimethoxyphenyl)bismuth composition in a weight ratio of 1:1 as the condensing agent mixed. Insulate at 40-43°C for reaction, monitor by TLC, TLC shows that the reaction is complete, after treatment, 87.4 kg (224 mol) of intermediate III was obtained, with a yield of 95.4%.
Embodiment 3
[0043] The difference between embodiment 3 and embodiment 1 is that a combined condensing agent is adopted, and the combined condensing agent is made of, and the condensing agent is 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and three (2 , 6-dimethoxyphenyl) bismuth combined, the weight percentage of tris (2,6-dimethoxyphenyl) bismuth in the condensing agent is 25%, heat preservation 18~22 ℃ reaction, TLC monitoring, after 88 kg (225.6 mol) of intermediate III was obtained with a yield of 96%.
[0044]The N,N'-carbonyldiimidazole in the combined condensing agent can be replaced by 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride, N,N'-carbonyldiimidazole, 1 Other combinations within the range of -hydroxybenzotriazole, 1-hydroxy-7-azobenzotriazole, and tris(2,6-dimethoxyphenyl)bismuth, the yields of which are all above 92%; the combination The condensing agent is composed of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride and tris(2,6-dimeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com