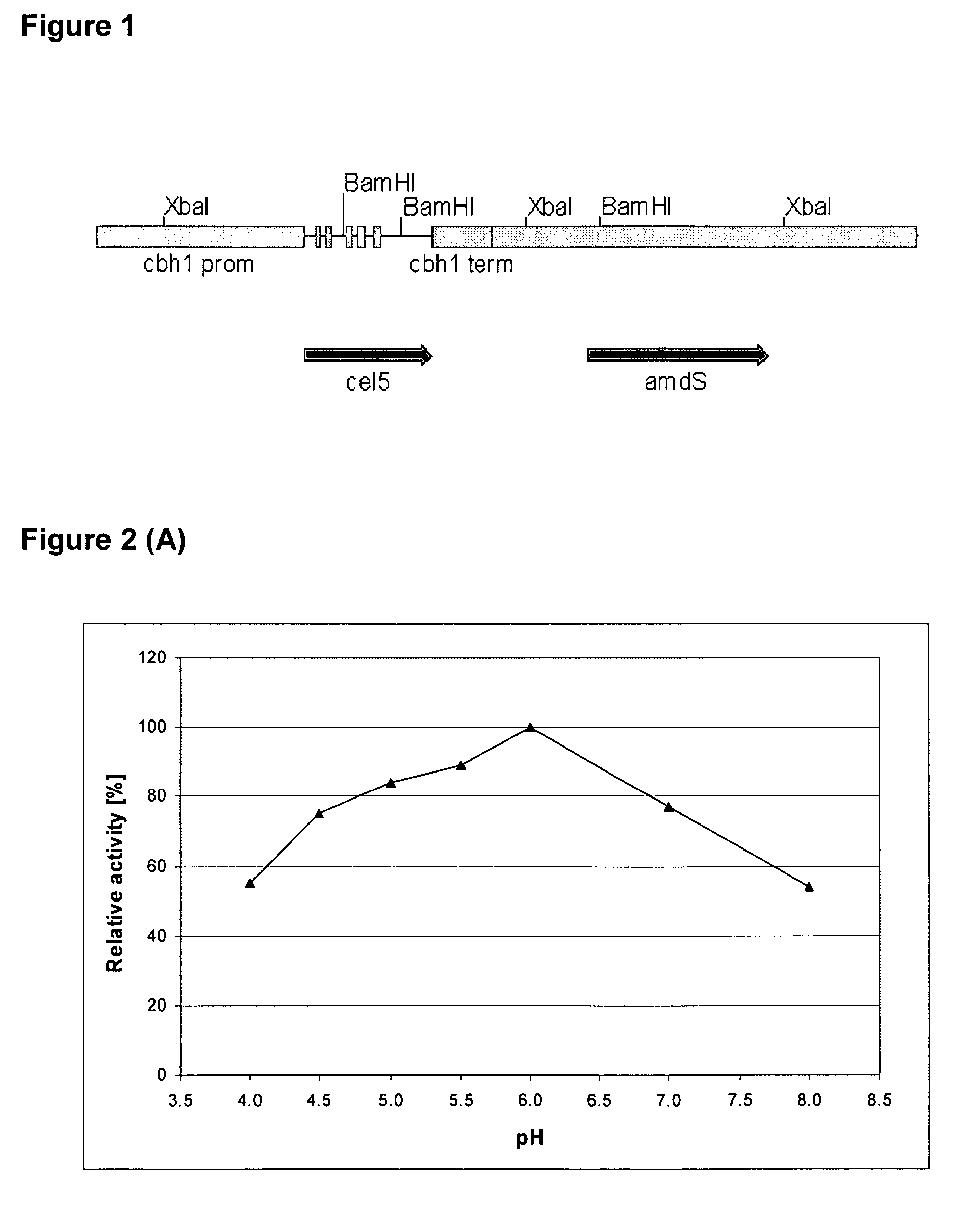
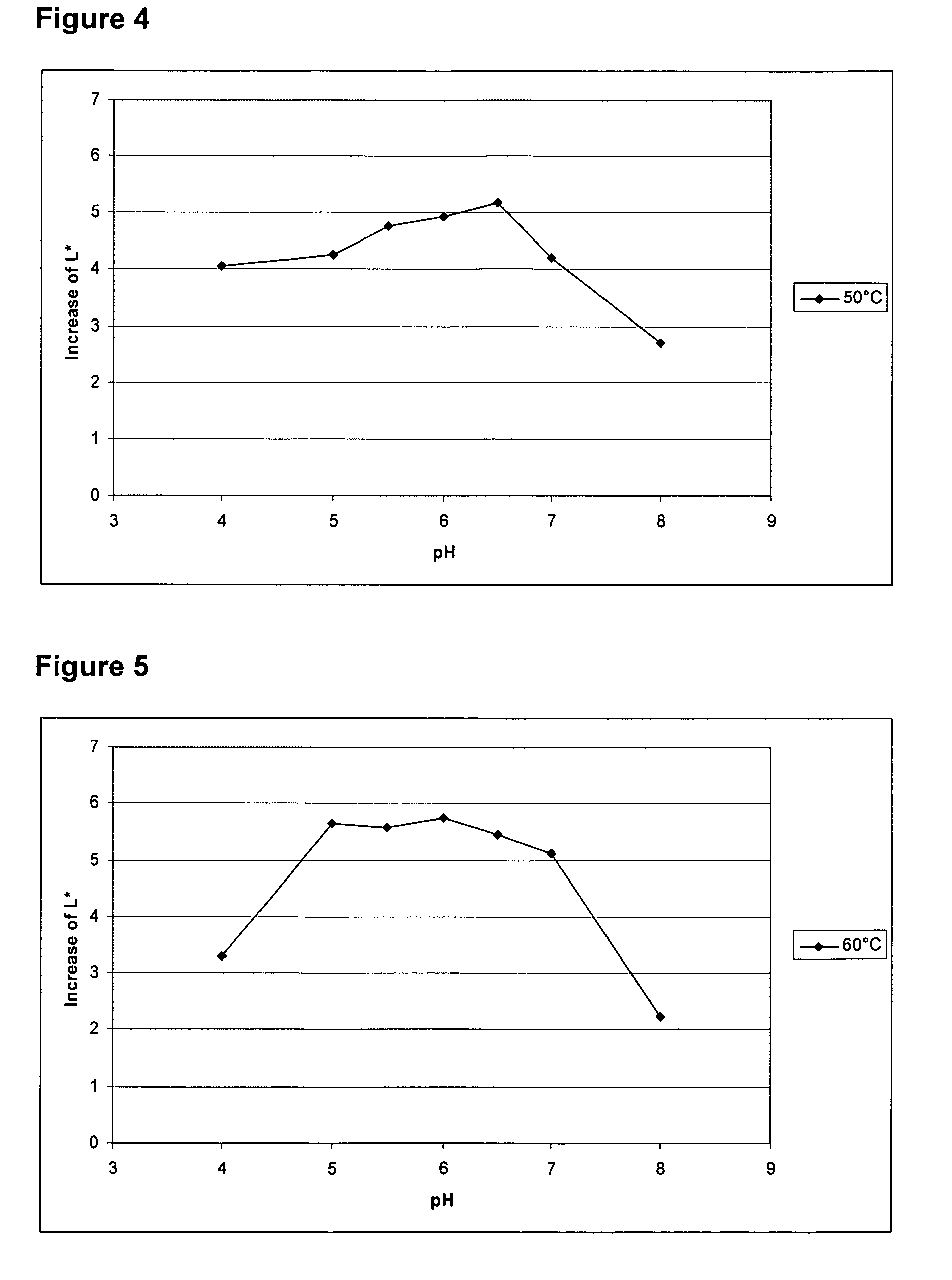
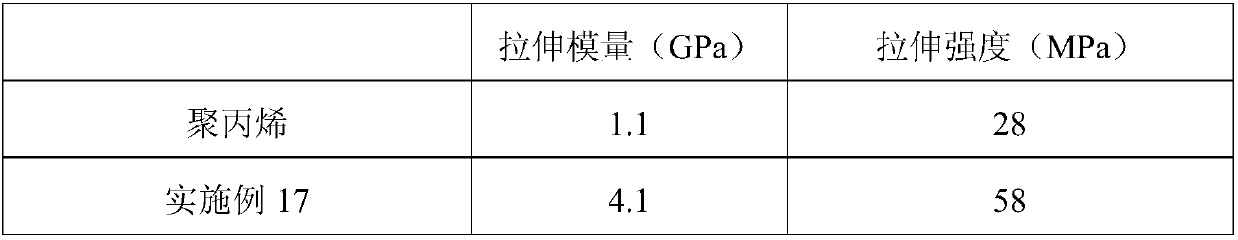
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
148 results about "Cellulose binding" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interacting selectively and non-covalently with cellulose. [GOC:mah]
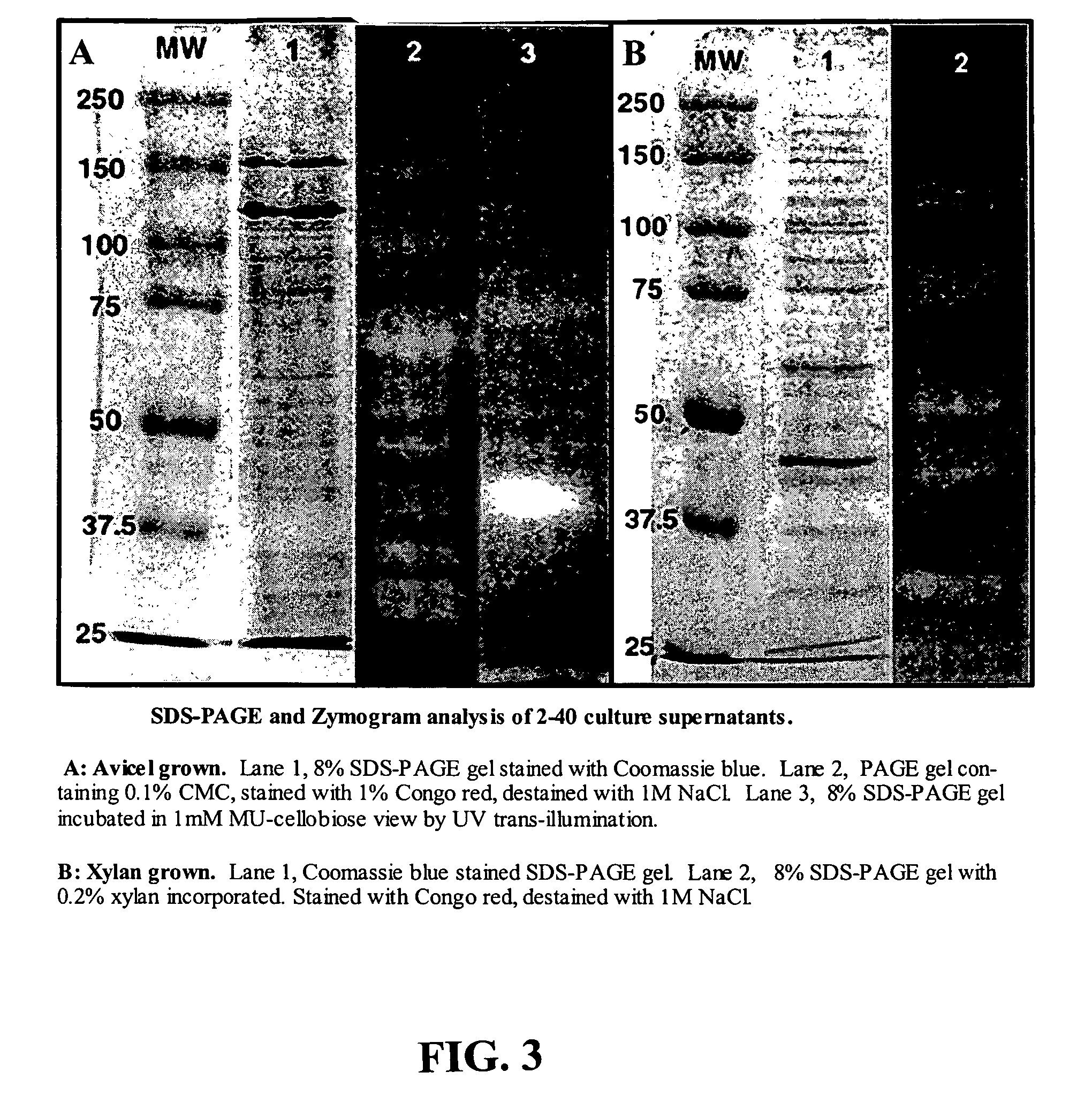
Construction of highly efficient cellulase compositions for enzymatic hydrolysis of cellulose
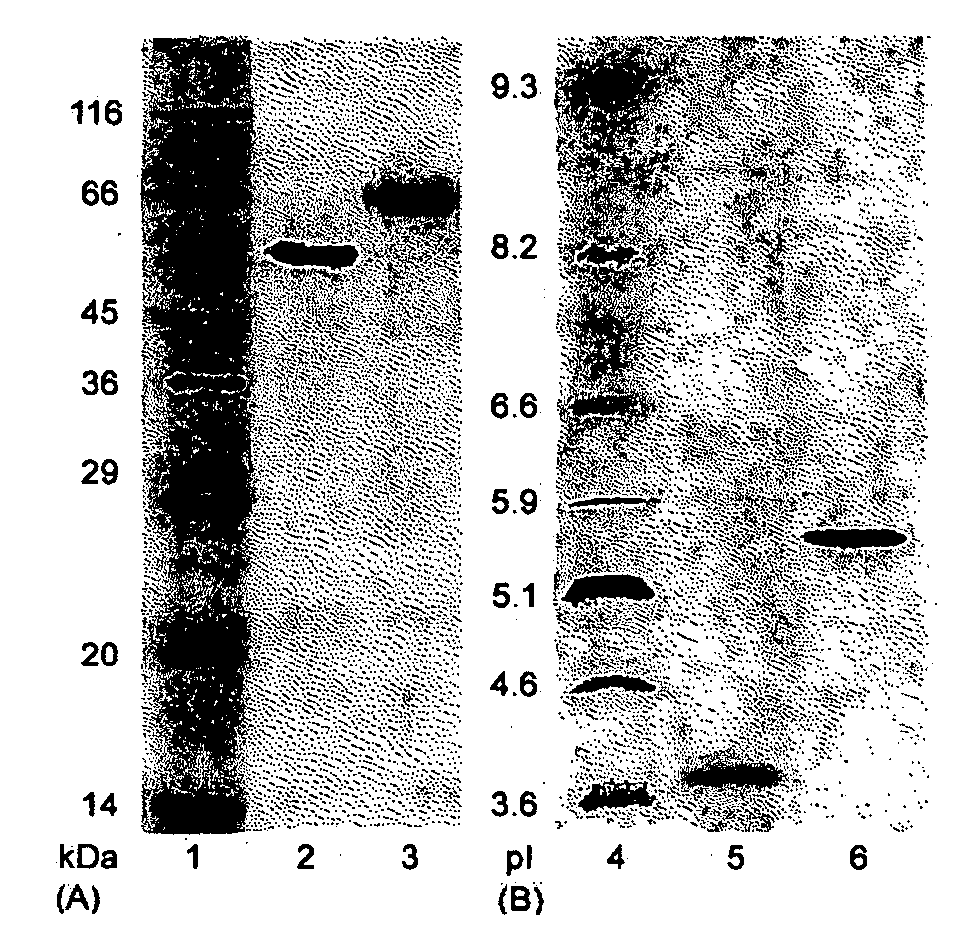
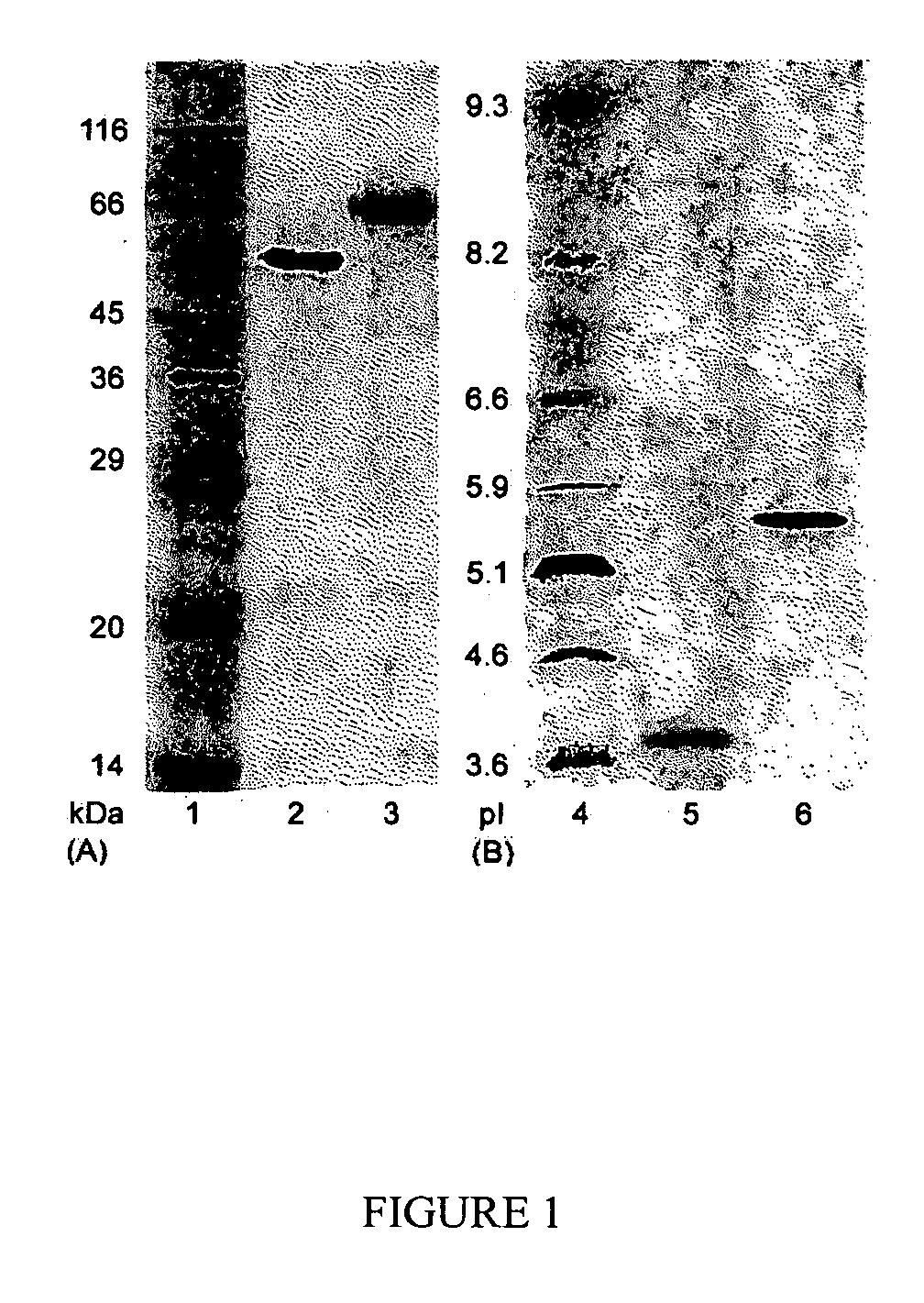
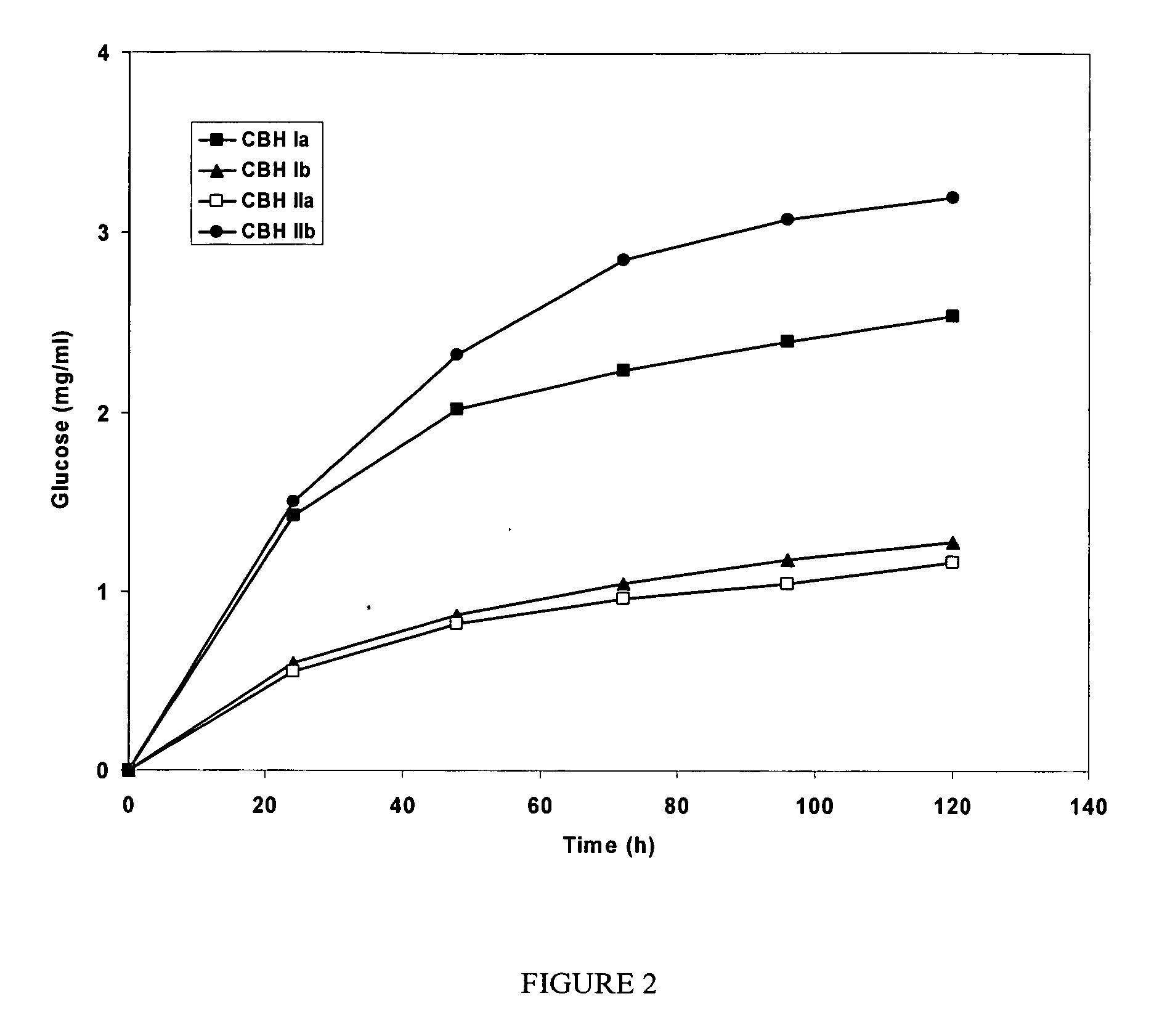
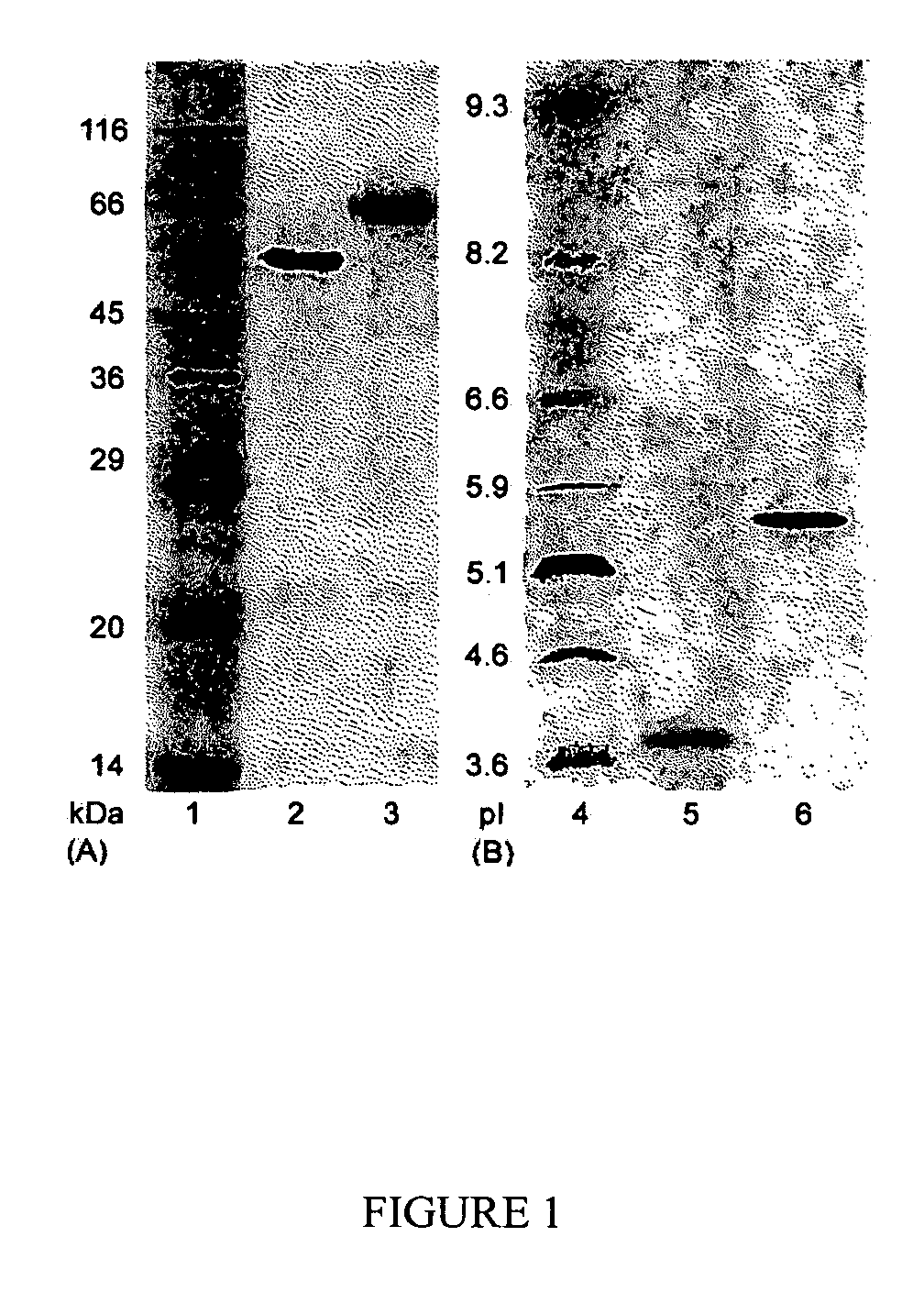
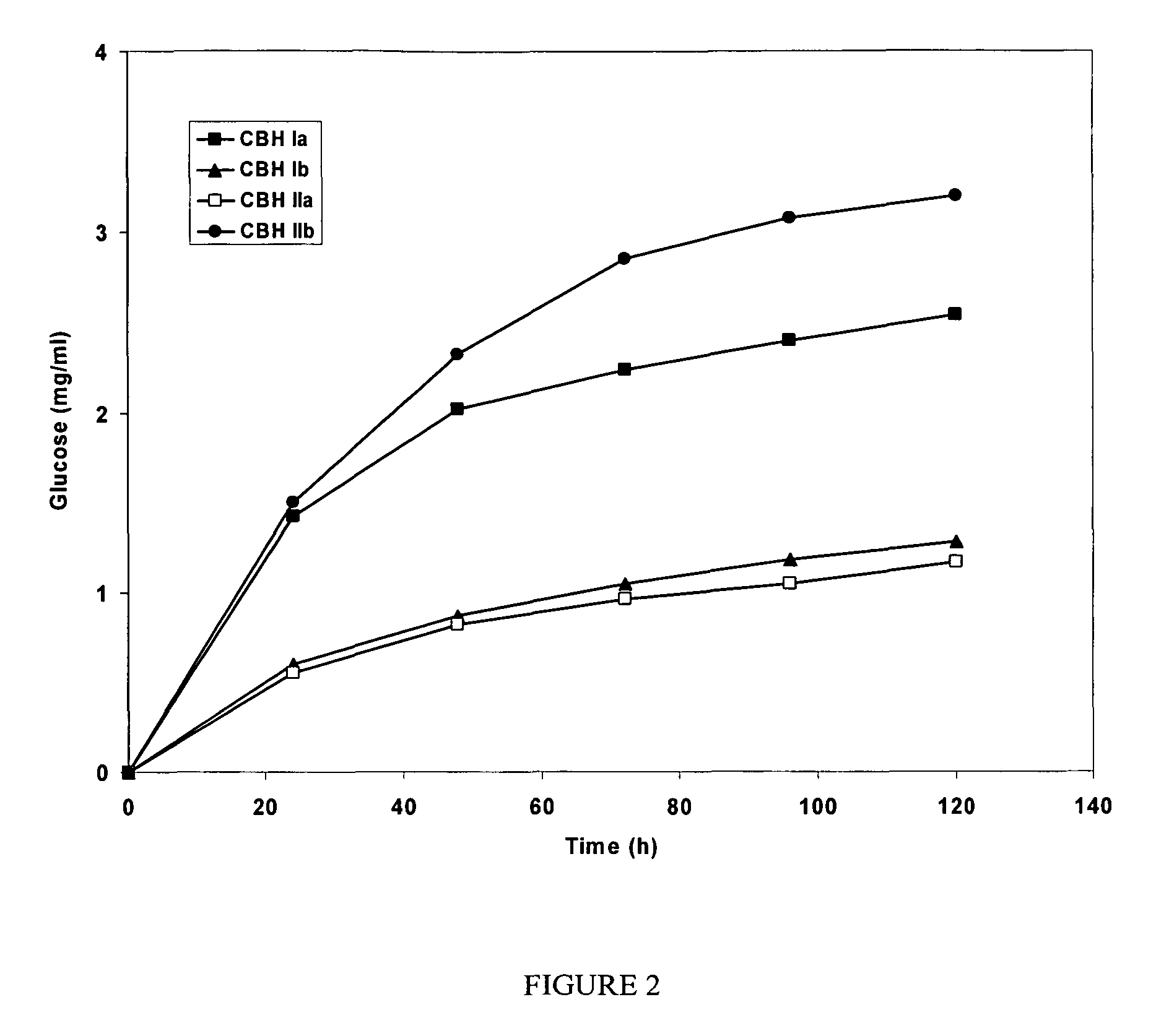
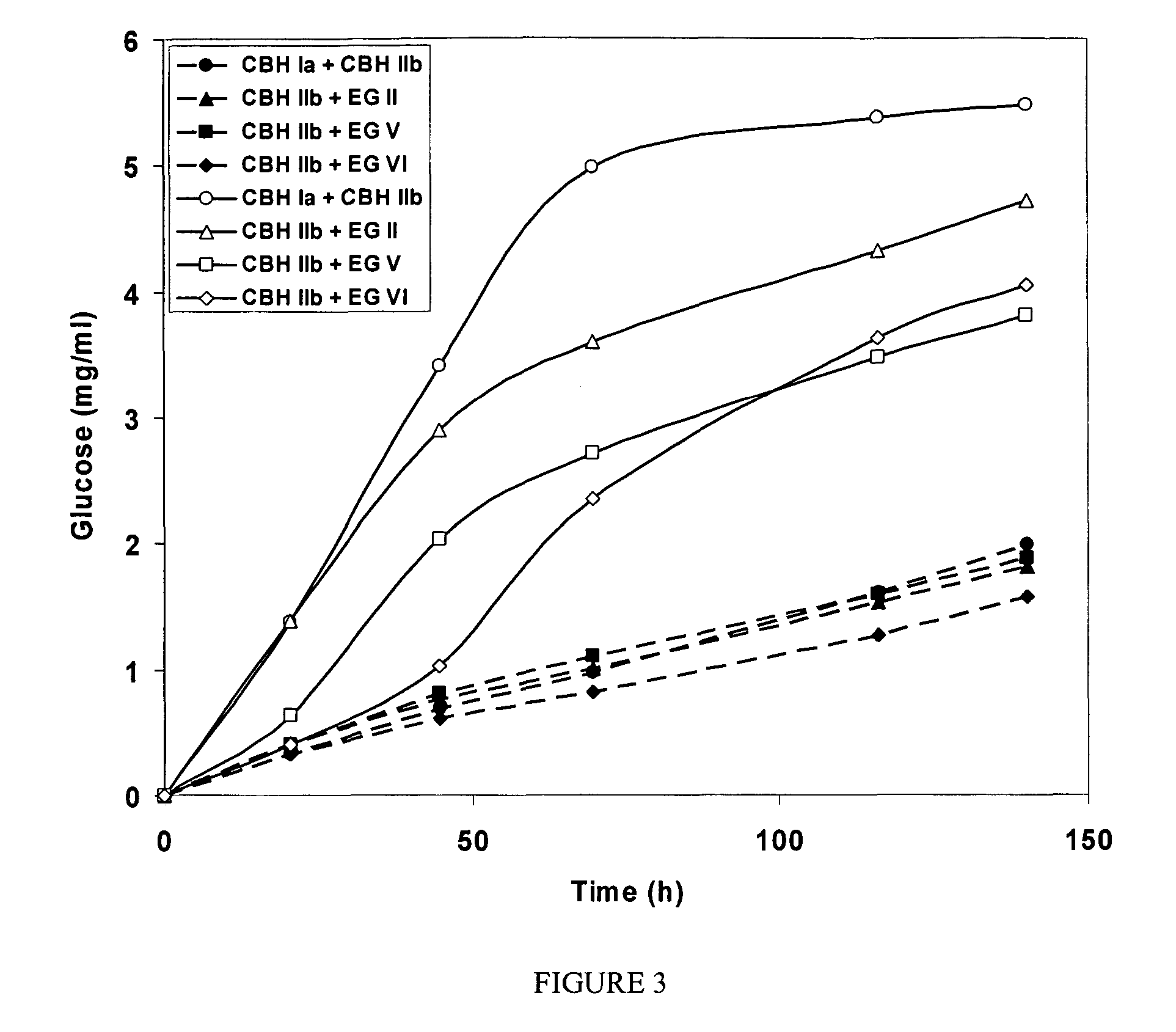
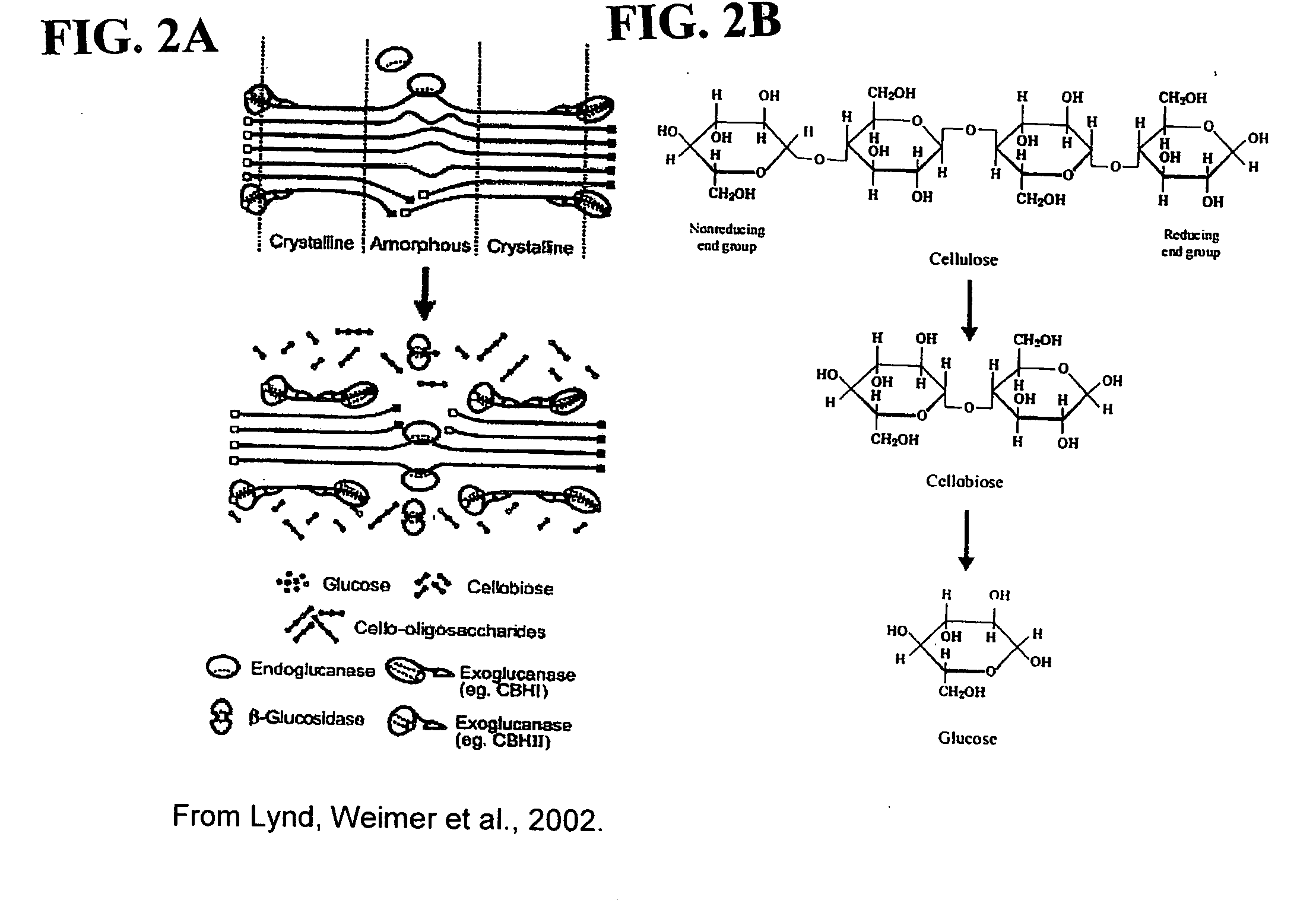
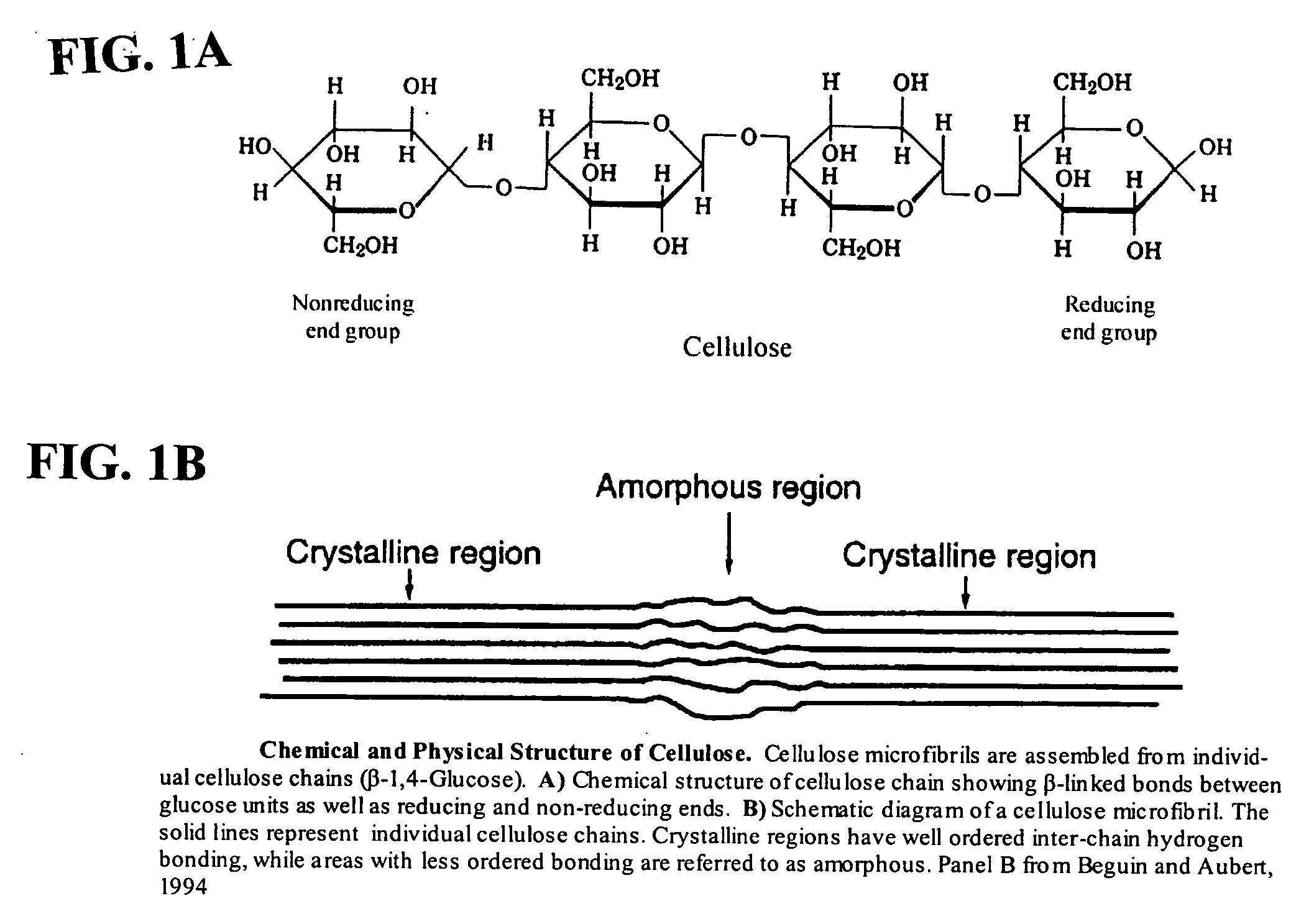
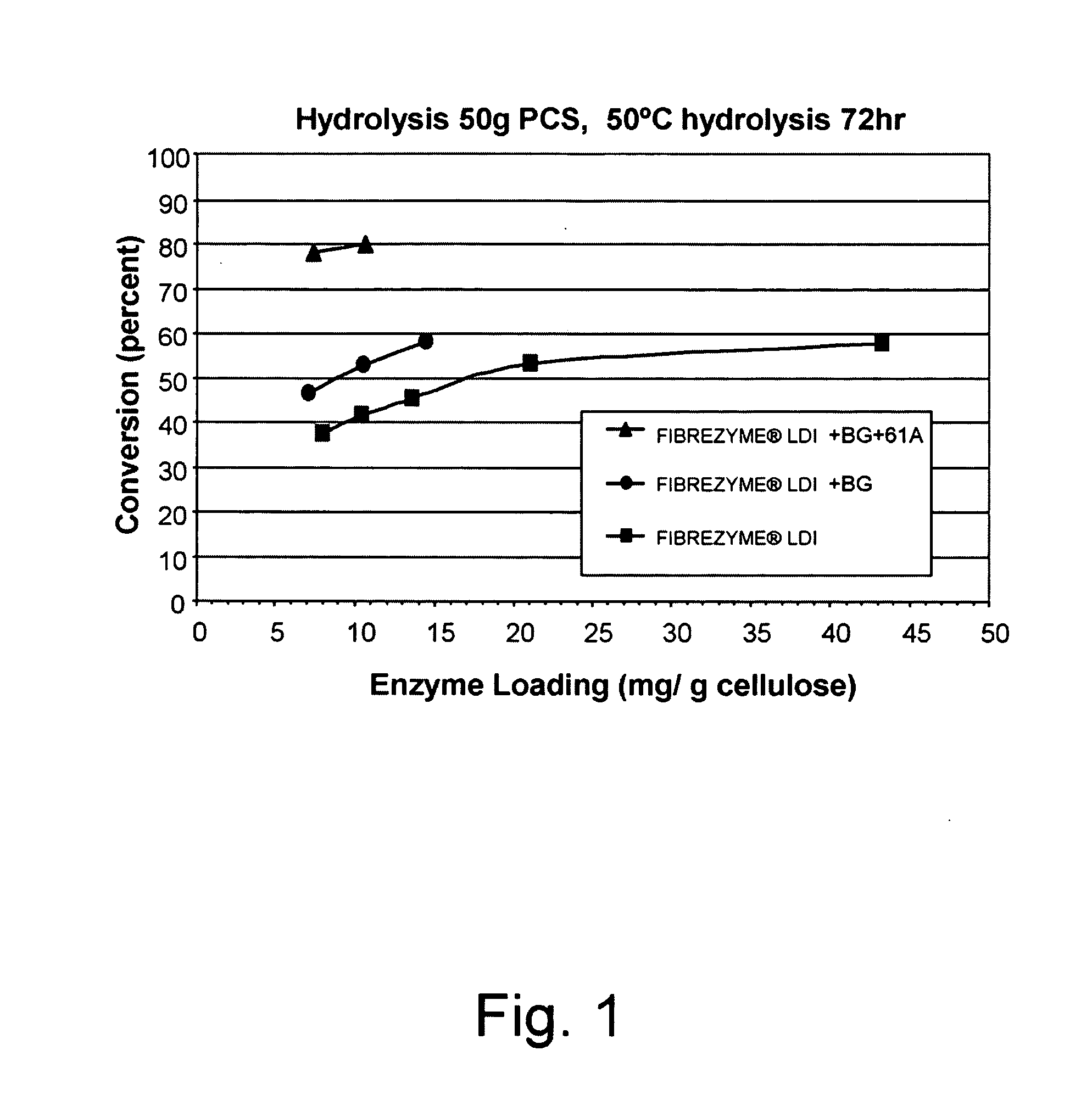
This invention provides novel enzyme compositions using newly identified and isolated C. lucknowense enzymes, including CBH Ib CBH IIb, EG II, EG VI, β-glucosidase, and xylanase II in conjunction with previously identified enzymes CBH Ia, CBH IIa (previously described as Endo 43), and EG V. These enzyme compositions demonstrate an extremely high ability to convert lignocellulosic biomass (e.g., Avicel, cotton, Douglas fir wood pretreated by organosolv) to glucose. CBH Ia and IIb, which both have a cellulose-binding module (CBM) displayed a pronounced synergism with three major endoglucanases (EG II, EG V, EG VI) from the same fungus in hydrolysis of cotton as well as a strong synergy with each other. The enzyme compositions are effective in hydrolysis of the lignocellulosic biomass.
Owner:DANISCO US INC
Conjugated polysaccharide fabric detergent and conditioning products
InactiveUS6225462B1Advantage of extraExtra compatibilityOrganic detergent compounding agentsSugar derivativesCellulose bindingLocust bean gum
A polysaccharide conjugate comprises a polysaccharide with an attached entity having a molecular weight of at least 5000, the polysaccharide conjugate being capable of binding to cellulose. Preferred polysaccharides include tamarind seed xyloglucan, locust bean gum and enzyme modified guar. The attached entity is suitably a protein such as an enzyme, antibody or antibody fragment, or a particle possibly having a benefit agent such as a fragrance associated therewith. Because the polysaccharide conjugate binds to cellulose, which is present in cotton and other fabrics, paper, etc., binding of the conjugate to cellulose brings the attached entity into close proximity to a surface of or containing cellulose. The invention thus enables targeting of attached entities to such surfaces. The invention also provides a product incorporating the polysaccharide conjugate of the invention. The product is conveniently a laundry product such as a fabric washing product, e.g. a detergent product, or a fabric conditioning product. In this case the attached entity may be an enzyme, a particle bearing fragrance, etc. The invention also provides a method of targeting binding of an entity to cellulose by use of the polysaccharide conjugate of the invention.
Owner:LEVER BROTHERS
Construction of highly efficient cellulase compositions for enzymatic hydrolysis of cellulose
InactiveUS7883872B2High activityPronounced synergismBryophytesSugar derivativesCellulose bindingEnzymatic hydrolysis
This invention provides novel enzyme compositions using newly identified and isolated C. lucknowense enzymes, including CBH Ib CBH IIb, EG II, EG VI, β-glucosidase, and xylanase II in conjunction with previously identified enzymes CBH Ia, CBH IIa (previously described as Endo 43), and EG V. These enzyme compositions demonstrate an extremely high ability to convert lignocellulosic biomass (e.g., Avicel, cotton, Douglas fir wood pretreated by organosolv) to glucose. CBH Ia and IIb, which both have a cellulose-binding module (CBM) displayed a pronounced synergism with three major endoglucanases (EG II, EG V, EG VI) from the same fungus in hydrolysis of cotton as well as a strong synergy with each other. The enzyme compositions are effective in hydrolysis of the lignocellulosic biomass.
Owner:DANISCO US INC
Polishing pad for use in chemical-mechanical planarization of semiconductor wafers and method of making same
InactiveUS6852020B2Prolong lifeSolution to short lifeOther chemical processesAbrasion apparatusFiberSlurry transport
A polishing pad for use in chemical mechanical polishing of substrates that being made of a porous structure comprising a matrix consisting of fibers, such as cotton linter cellulose bound with a thermoset resin, such as phenolic resin. The polishing pad surface has voids in which polishing slurry flows during chemical mechanical polishing of substrates, and in which debris formed during the chemical-mechanical polishing of substrates is temporarily stored for subsequent rinsing away. The polishing surface of the pad is ground to form asperities that aid in slurry transport and polishing, as well as opening the porous structure of the pad. The porous pad contains nanometer-sized filler-particles that reinforce the structure, imparting an increased resistance to wear as compared to prior-art pads. Also disclosed is a method of making the polishing pad.
Owner:RAYBESTOS POWERTRAIN
Process of expressing and isolating recombinant proteins and recombinant protein products from plants, plant derived tissues or cultured plant cells
A process of expressing a recombinant protein in a plant and of isolating the recombinant protein from the plant, the process is effected by (a) providing a plant, a plant derived tissue or cultured plant cells expressing a fusion protein including the recombinant protein and a cellulose binding peptide being fused thereto, the fusion protein being compartmentalized within cells of the plant, plant derived tissue or cultured plant cells, so as to be sequestered from cell walls of the cells of the plant, plant derived tissue or cultured plant cells; (b) homogenizing the plant, plant derived tissue or cultured plant cells, so as to bring into contact the fusion protein with a cellulosic matter of the plant, plant derived tissue or cultured plant cells, to thereby effect affinity binding of the fusion protein via the cellulose binding peptide to the cellulosic matter, thereby obtaining a fusion protein cellulosic matter complex; and (c) isolating the fusion protein cellulosic matter complex.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Enzyme systems for saccharification of plant cell wall polysaccharides
The present invention relates to cell wall degradative systems, in particular to systems containing enzymes that bind to and / or depolymerize cellulose. These systems have a number of applications. Some embodiments relate to a method of producing ethanol using the cell wall degradative systems of the present invention.
Owner:UNIV OF MARYLAND
Alginases, systems containing alginases and methods of cloning, purifying and/or utilizing alginases
Owner:UNIV OF MARYLAND
Isolated polypeptide having arabinofuranosidase activity
Owner:DANISCO US INC
Method for pretreating cotton fabrics by adopting glucoamylase/glucose oxidase, pectinase and CBD (cellulose-binding domain)-lignin peroxidase
InactiveCN102154810AImprove qualityImprove dyeing qualityBiochemical fibre treatmentDry-cleaning apparatus for textilesChemical treatmentPectinase
The invention provides a method for pretreating cotton fabrics by adopting glucoamylase / glucose oxidase, pectinase and CBD (cellulose-binding domain)-lignin peroxidase, belonging to the technical field of cotton fabric dyeing and finishing pretreatment. The method is characterized in that the cotton fabrics are firstly treated in mixed enzyme solution containing the glucoamylase and the glucose oxidase and the glucoamylase catalyzes the starch size on the fabrics to be decomposed into glucose so as to eliminate the size; the glucose oxidase catalyzes the glucose and oxygen to act to generate hydrogen peroxide; the generated hydrogen peroxide not only plays a role of bleaching but also can damage the hydrophobic surface structure of the cotton to expose the lower pectic substances; then the cotton fabrics are treated in alkaline pectinase solution to remove the pectic substances through decomposition; and finally the cotton fabrics are treated in CBD-lignin peroxidase solution to finally remove the cotton seed hulls through decomposing the lignin in the cotton seed hulls. The method provided by the invention can replace the traditional chemical treatment method to realize full bio-enzyme pretreatment of the cotton fabrics, and at the same time the traditional hydrogen peroxide can be no longer adopted for bleaching.
Owner:JIANGNAN UNIV +1
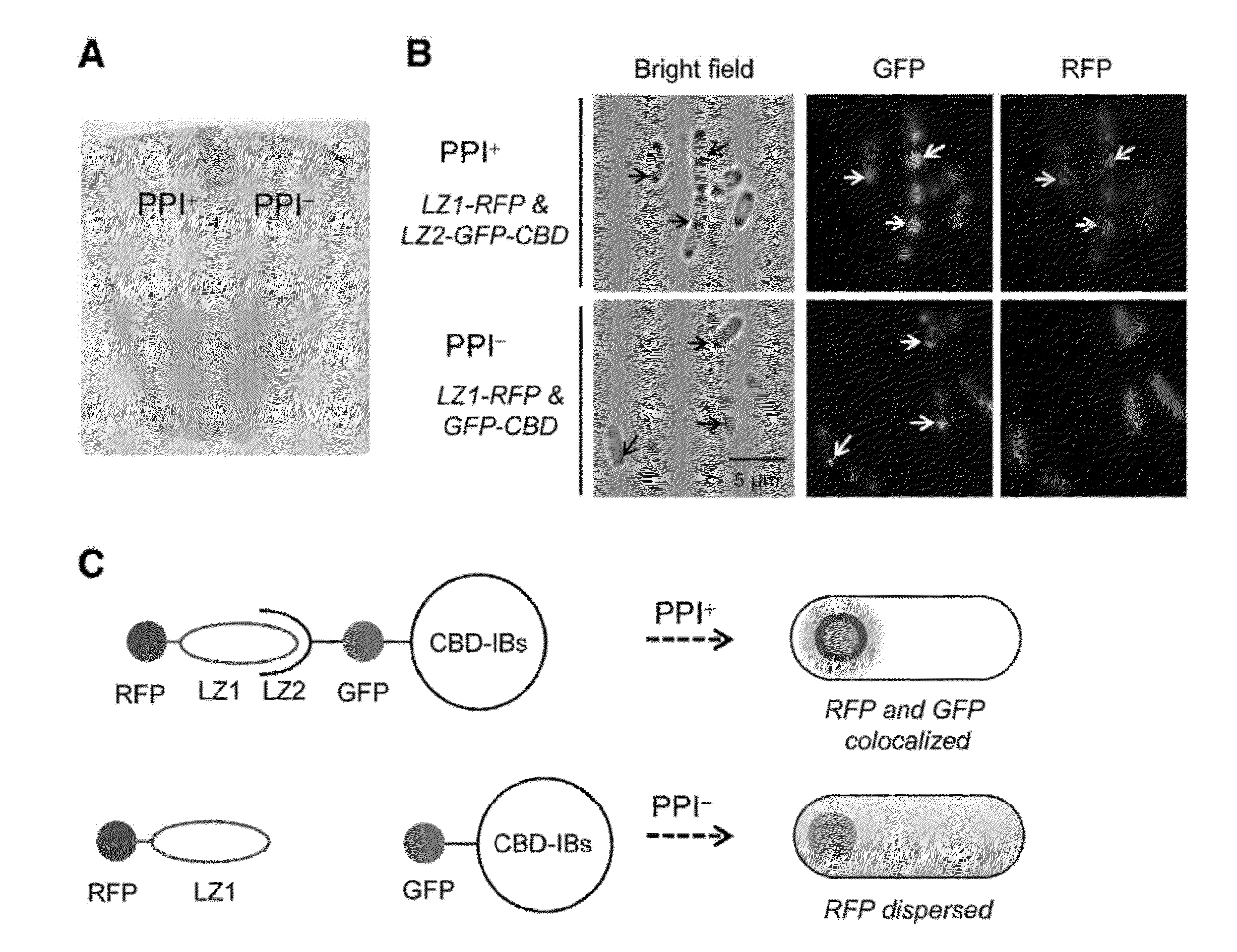
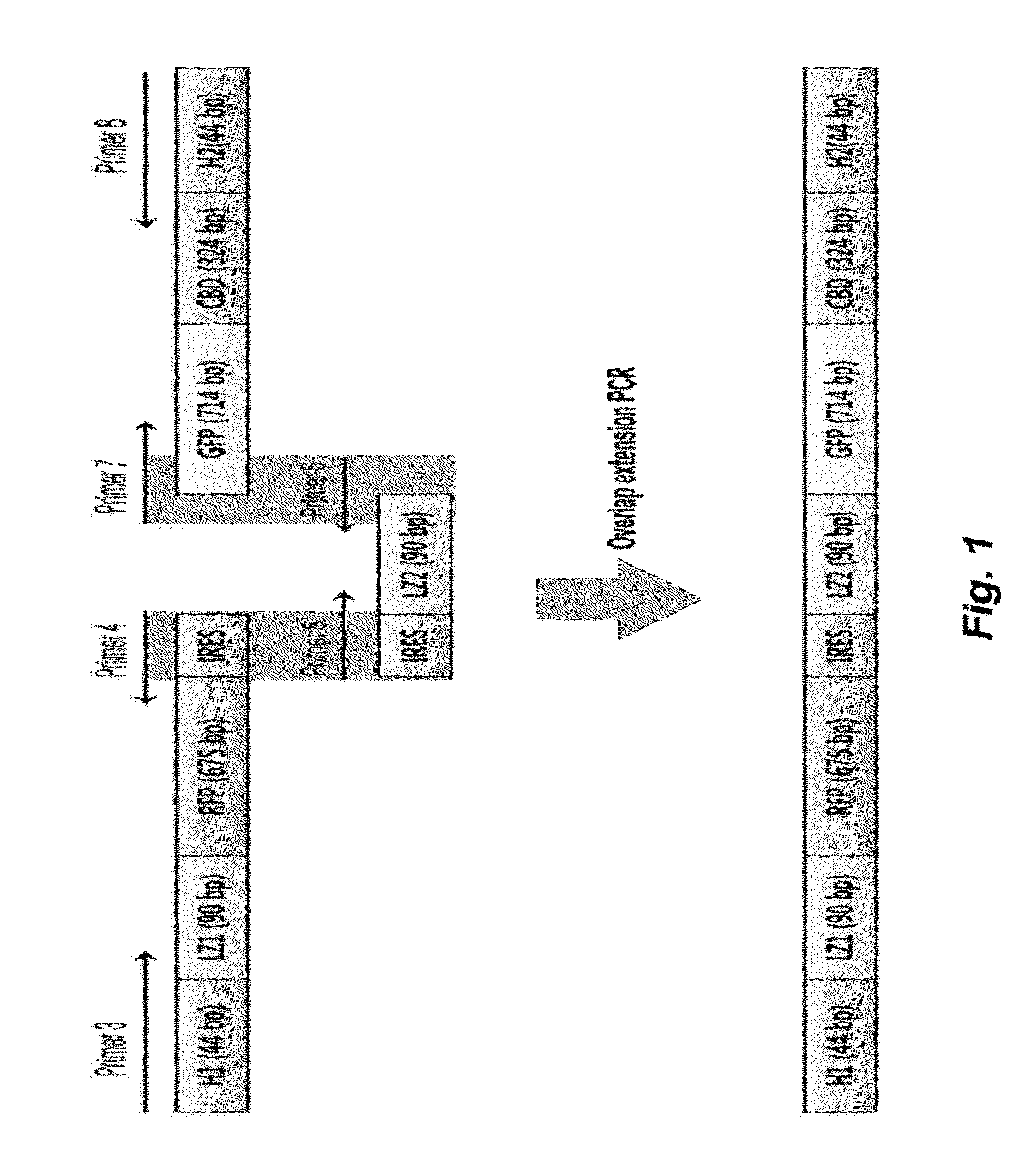
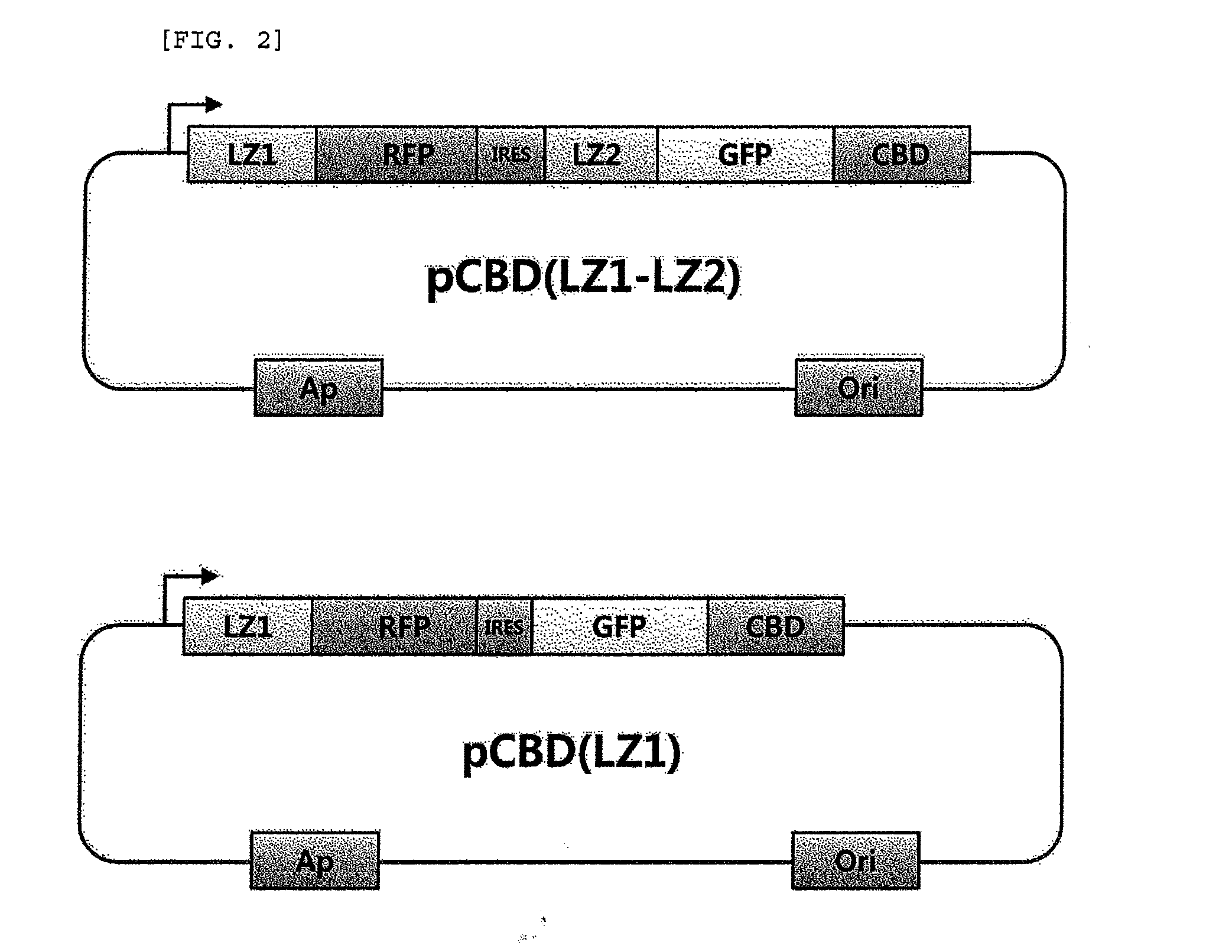
Method for detecting protein-protein interactions in cells
The present invention relates to a method for detecting protein-protein interactions in living cells, and more particularly, to a method for providing cells comprising a first construct and a second construct, wherein the first construct comprises a polynucleotide encoding a first fusion protein which comprises a bait protein, a first fluorescent protein and a CBD (cellulose-binding domain) protein, and wherein the second construct comprises a polynucleotide encoding a second fusion protein which comprises a prey protein and a second fluorescent protein so as to allow formation of inclusion bodies, and detecting interactions between the bait protein and the prey protein that are displayed by inclusion bodies, a method for isolating the prey protein bound to the bait protein using the cells comprising the constructs, the cells, and a kit for detecting protein-protein interactions, comprising the constructs.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Method of treating fabrics and apparatus used therein
InactiveUS6586384B2Organic detergent compounding agentsNon-surface-active detergent compositionsCelluloseCellulose binding
Owner:HENKEL IP & HOLDING GMBH
Novel trichoderma genes
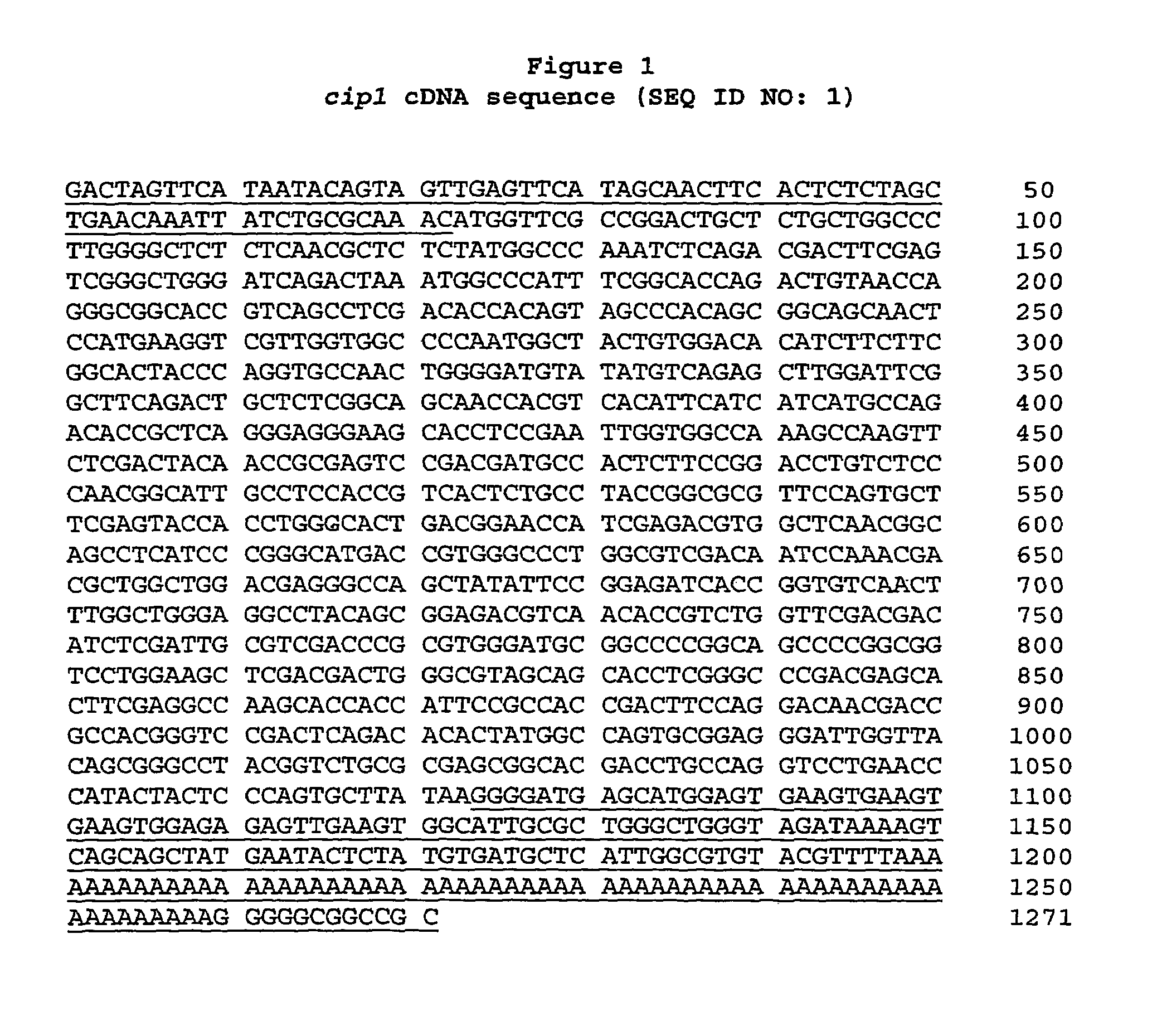
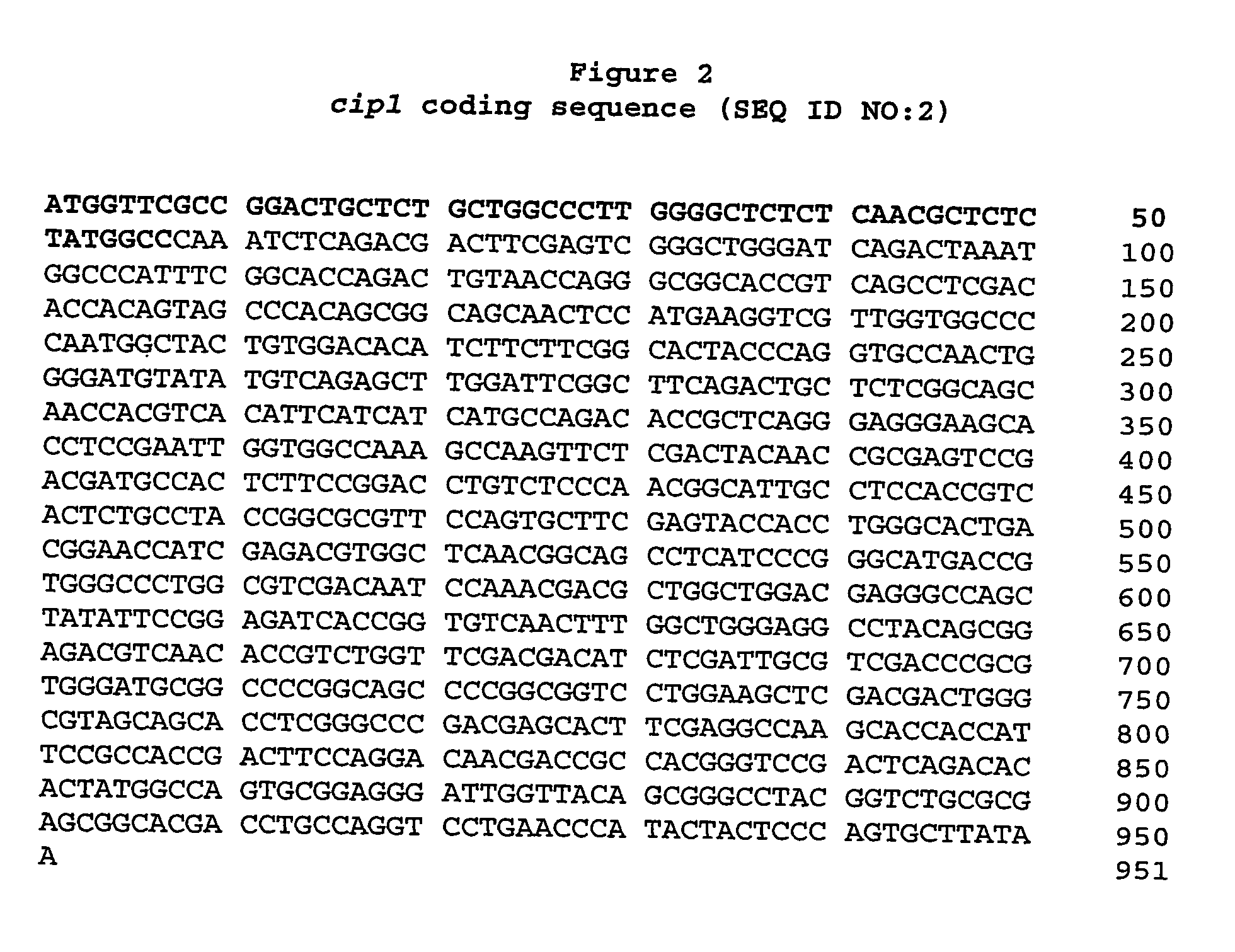
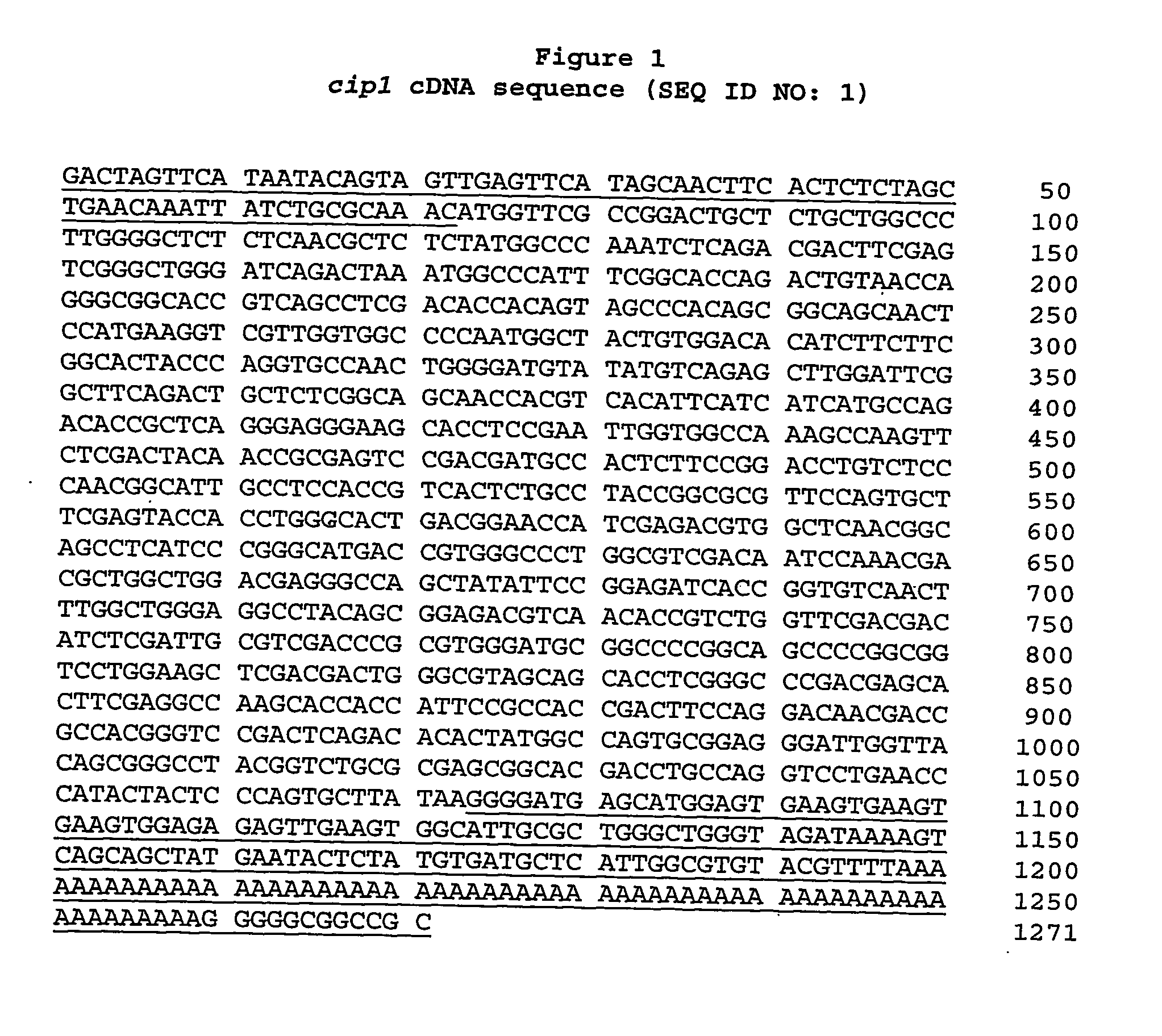
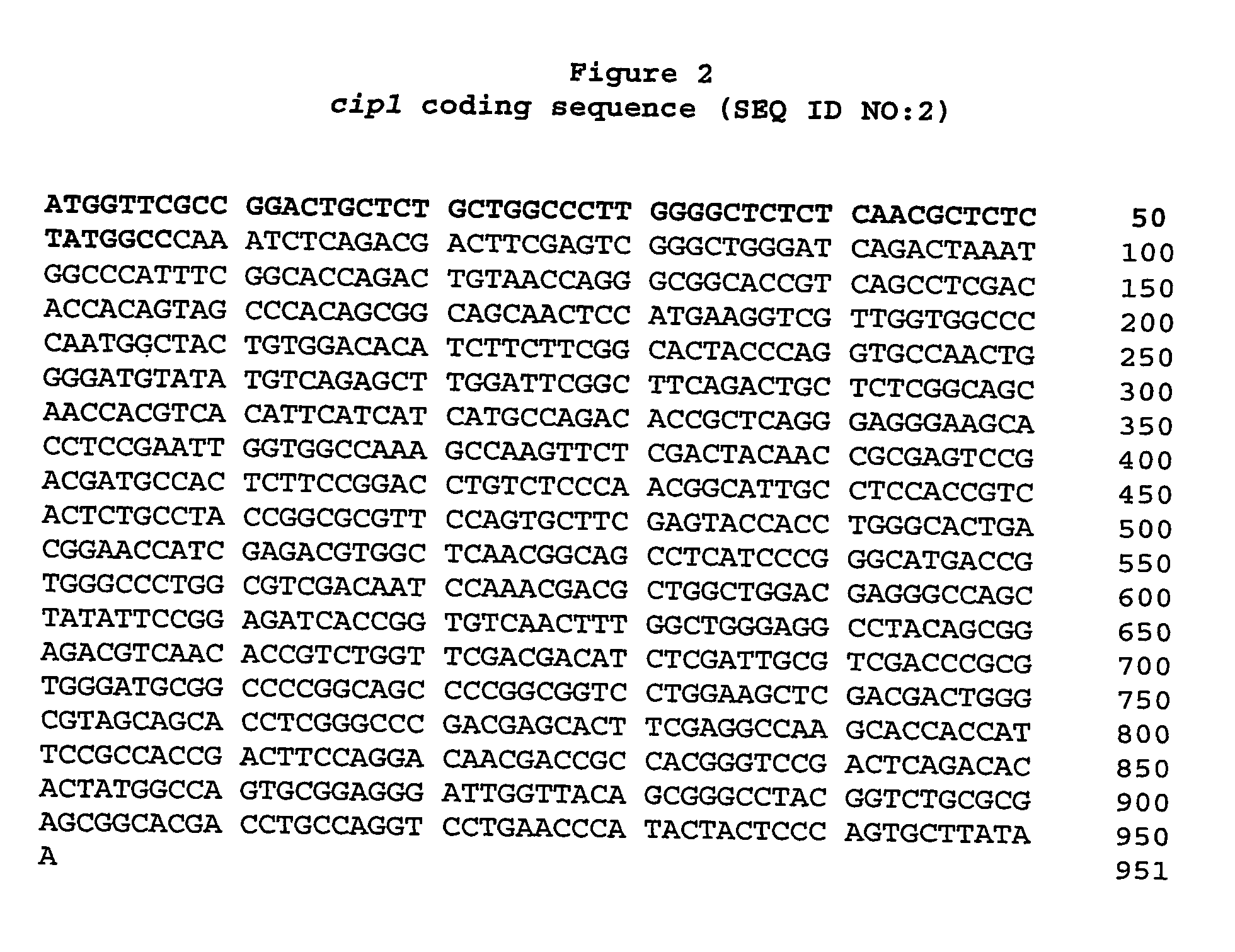
Described herein are novel gene sequences isolated from Trichoderma reesei. Two genes encoding proteins comprising a cellulose binding domain, one encoding an arabionfuranosidase and one encoding an acetylxylanesterase are described. The sequences, CIP1 and CIP2, contain a cellulose binding domain. These proteins are especially useful in the textile and detergent industry and in pulp and paper industry.
Owner:DANISCO US INC
Method of treating fabrics
InactiveUS6579842B2Cosmetic preparationsOrganic detergent compounding agentsCelluloseCellulose binding
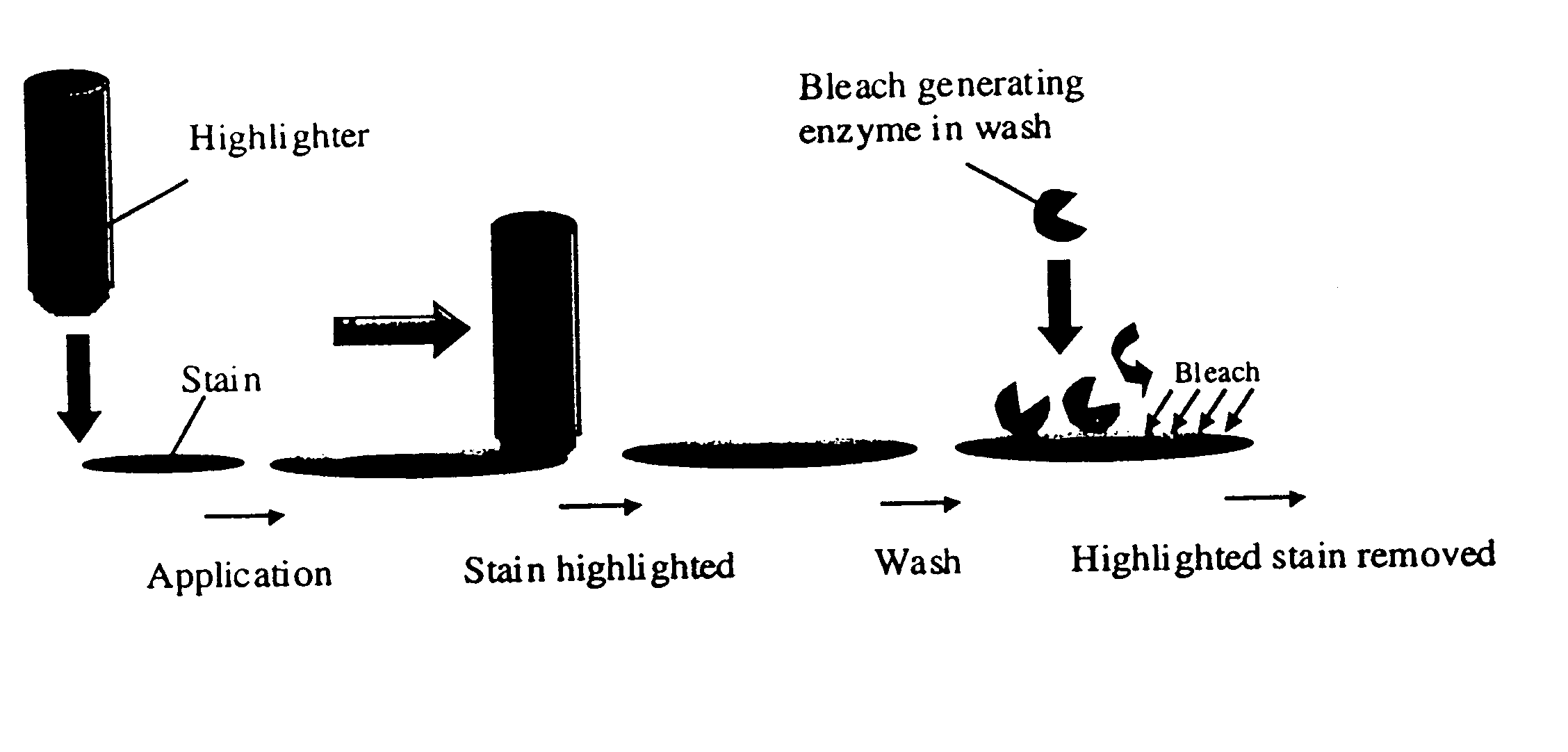
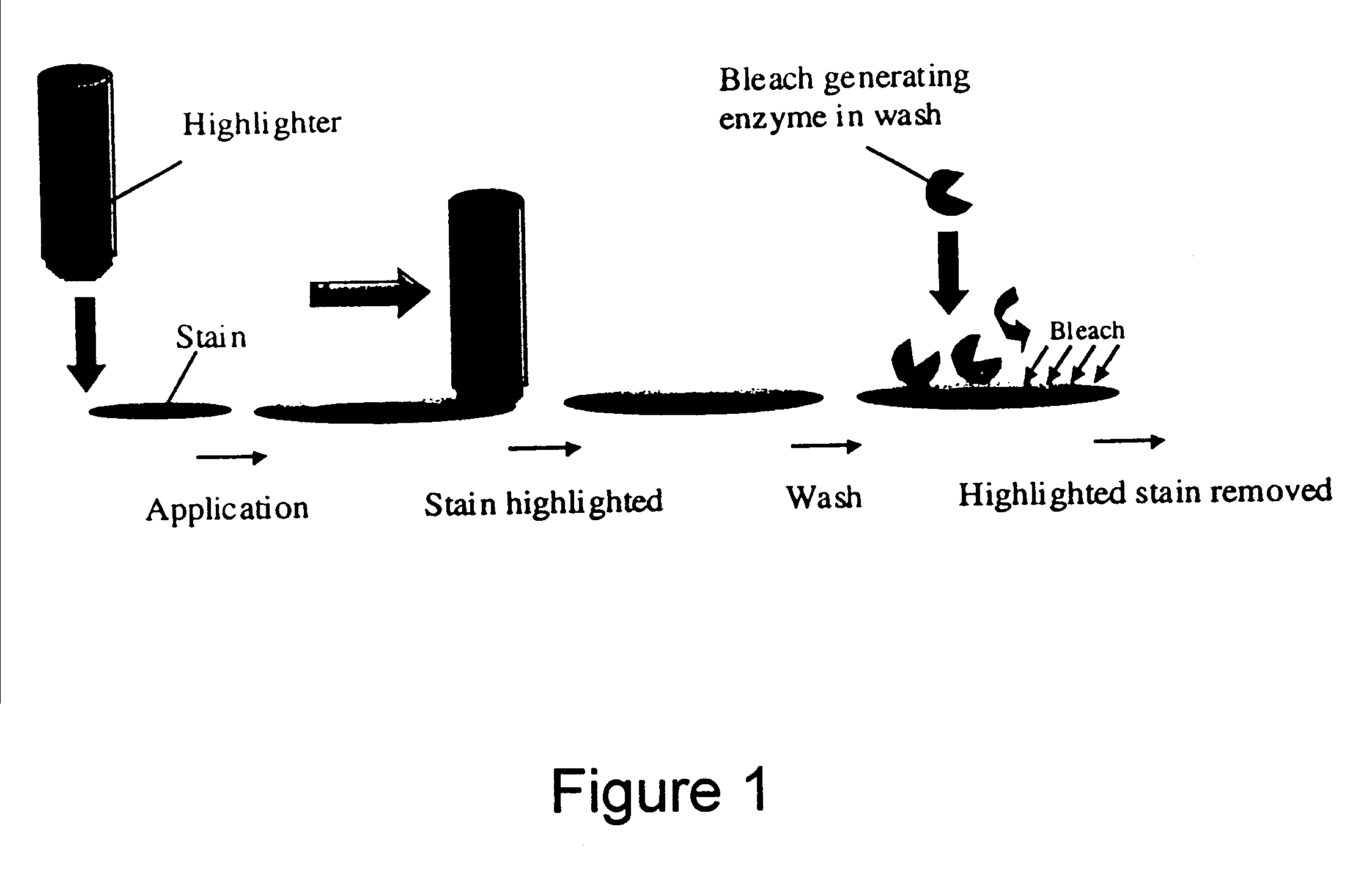
A method of delivering a benefit agent to fabric for exerting a pre-determined activity, wherein the fabric is pre-treated with a multi-specific binding molecule which has a high binding affinity to the fabric through one specificity and is capable of binding to the benefit agent through another specificity, followed by contacting the pre-treated fabric with the benefit agent, to enhance the pre-determined activity to the fabric. Preferably, the binding molecule is an antibody or fragment thereof, or a fusion protein comprising a cellulose binding domain and a domain having a high binding affinity to another ligand which is directed to said benefit agent. The method is useful for example for stain removal, perfume delivery, and treating collars and cuffs for wear.
Owner:HENKEL IP & HOLDING GMBH
Compositions and methods for enhancing the degradation or conversion of cellulose-containing material
The present invention relates to methods for degrading or converting a cellulose-containing material, comprising: treating the cellulose-containing material with an effective amount of a cellulolytic enzyme composition comprising a polypeptide having cellulolytic enhancing activity, and one or more (several) components selected from the group consisting of a CEL7 polypeptide having endoglucanase activity, a CEL12 polypeptide having endoglucanase activity, a CEL45 polypeptide having endoglucanase activity, a CEL7 polypeptide having cellobiohydrolase activity with a cellulose binding domain, and a CEL7 polypeptide having cellobiohydrolase activity without a cellulose binding domain. The present invention also relates to such cellulolytic enzyme compositions.
Owner:NOVOZYMES INC
High glucose resistance beta-glucosidase-CBD fusion enzyme, and expression gene and application thereof
ActiveCN103045565AImprove bindingEfficient degradationBacteriaMicroorganism based processesHigh concentrationFiber
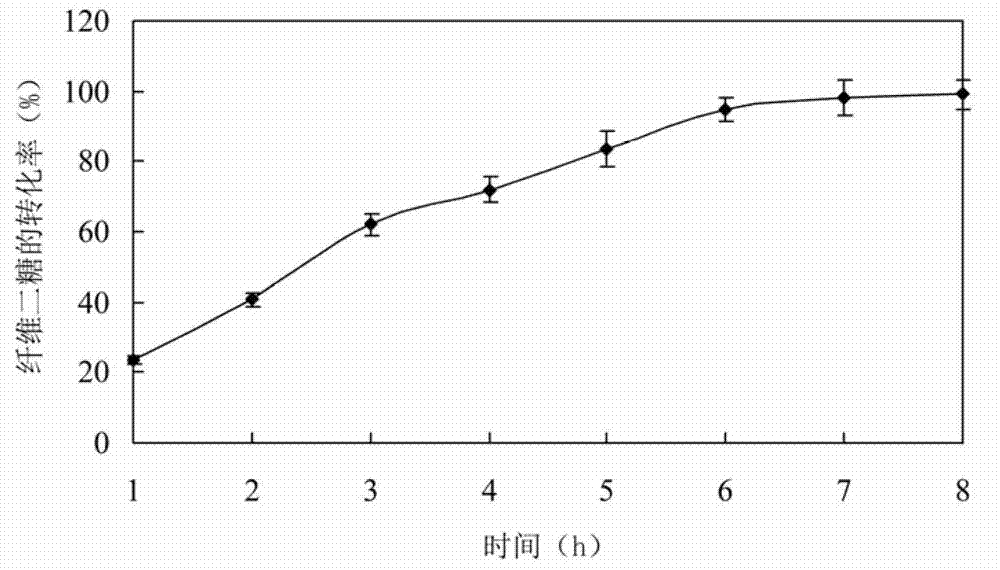
The invention relates to a high glucose resistance beta-glucosidase-CBD fusion enzyme, and expression gene and application thereof. The amino acid sequence is shown as SEQ ID NO. 1. Compared with the conventional beta-glucosidase enzyme, the fusion enzyme (BGL-CBD) provided by the present invention has the following characteristics: (1) higher cellulose binding ability, wherein the fusion enzyme (1 [mu]g / [mu]L) is incubated with 4 wt% of microcrystalline cellulose for 30min, and 98% of fusion enzyme is adsorbed by cellulose; (2) effectively degrading cellobiose, wherein the degradation rate of 10 wt% of the cellobiose by the fusion enzyme (0.15 [mu]g / [mu]L) within 8 h is 95%; and (3) resistance for high concentration glucose, wherein the glucose tolerance factor Ki is 1000 mmol / L.
Owner:NANJING FORESTRY UNIV
Enzyme fusion proteins and their use
ActiveUS20070244020A1Economical to provideEconomical to useBacteriaSugar derivativesDenimCellulose binding
Cellulase fusion proteins comprising an endoglucanase core region and a heterologous cellulose binding domain are described. The fusion proteins may be produced by recombinant techniques using appropriate polynucleotides, expressing vectors and host cells. The fusion proteins and enzyme preparations thereof are useful in treating cellulosic material, such as textile material, and they are particularly useful in biostoning denim or in biofinishing fabrics and garments. In addition the fusion proteins may be used in pulp and paper industry, oil extraction from plants, detergent compositions, or for improving the quality of animal feed.
Owner:AB ENZYMES OY
Method for preparing heat-resistant cutinase-CBD and application thereof in cotton fiber refining
ActiveCN101591648AEfficient expressionBiochemical fibre treatmentMicroorganism based processesCelluloseBiotechnology
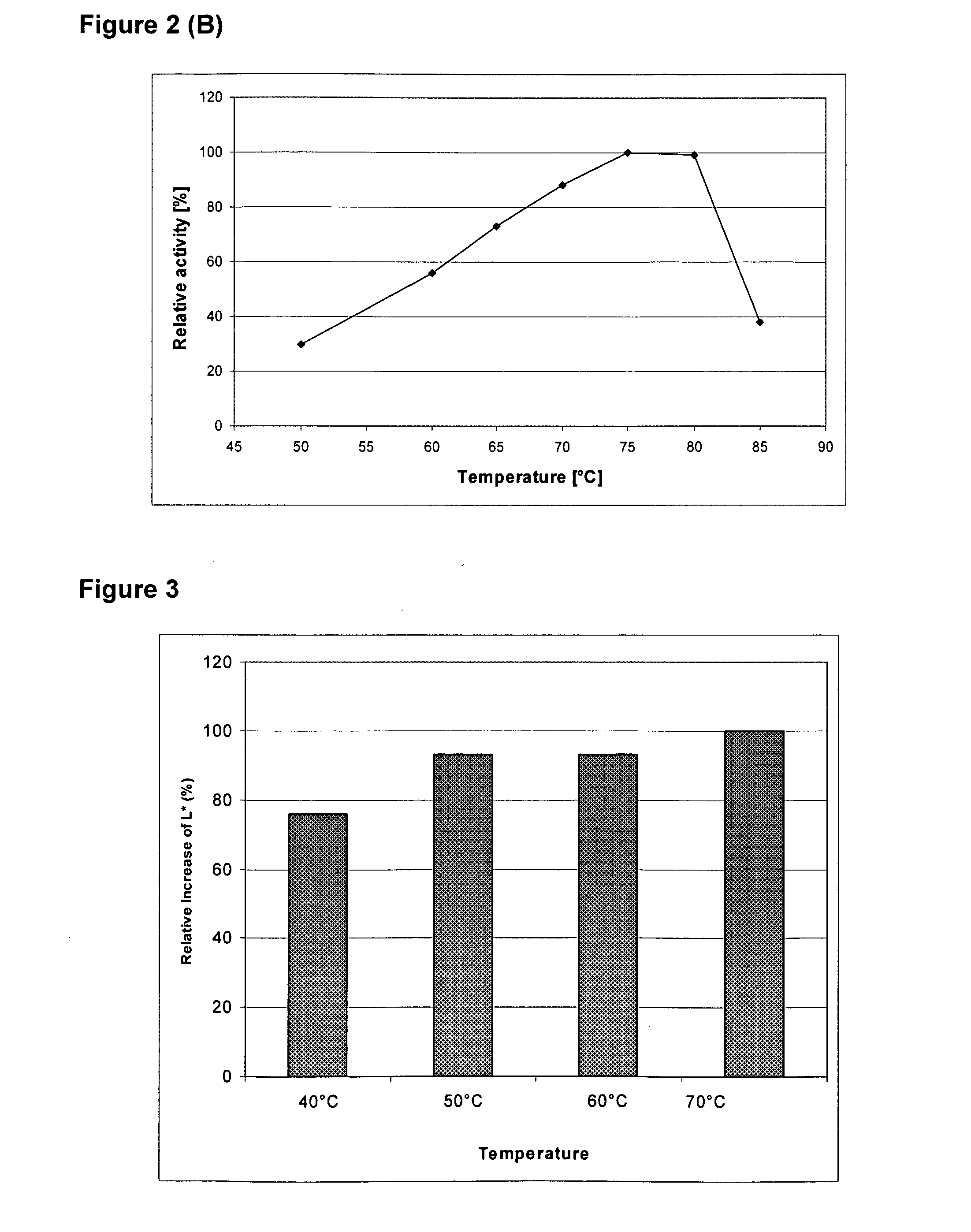
The invention discloses a method for preparing heat-resistant cutinase-CBD and application thereof to cotton fiber refining, which belong to the field of enzyme gene engineering. The method comprises the steps: taking pET20b-Tfu_0883 plasmid DNA and total DNA of thermophilic ascomycete WSH03-11 as a template, performing PCRs of designed primers respectively to obtain an encoding cutinase Tfu_0883 gene and a CBD gene in cellulose Tfu_1074, and then obtaining a fusion gene of encoding cutinase-cellulose-binding domain (CBD) (shortened as the heat-resistant cutinase-CBD) through overlap PCR; and taking pET20b(+) as an expression vector and E.coli BL21(DE3) as an expression host to realize the high level expression of a heat-resistant cutinase-CBD gene. The fusion enzyme has the activity of cutinase, the optimum temperature is 50 DEG C, the optimum pH value is 8, and the activity of the enzyme can still reach over 50 percent when heat preservation for 50h at a temperature of 50 DEG C. The cutinase-CBD has an absorption rate of 50 percent on cotton fiber, reaches reaction balance after 6h, and generates 180mu mol of fatty acid. The fusion enzyme is suitable for cotton fiber refining.
Owner:JIANGNAN UNIV
Recombinant cellulosome complex and uses thereof
ActiveUS9528132B2Degrading cellulose efficientlyEfficient degradationPolypeptide with localisation/targeting motifSugar derivativesSequence signalPleckstrin homology domain
The present invention relates to a polynucleotide encoding a recombinant scaffolding polypeptide comprising at least a signal peptide, a Cellulose Binding Domain, two cohesin domains and an S-layer Homology domain, wherein said isolated polynucleotide preferably comprises all or an active part of the nucleotide sequence as set forth in SEQ ID NO:2. The present invention further relates to vectors comprising such polynucleotides, recombinant lactic acid bacteria, and method for degrading a cellulosic biomass using such recombinant lactic acid bacteria.
Owner:CARBIOS
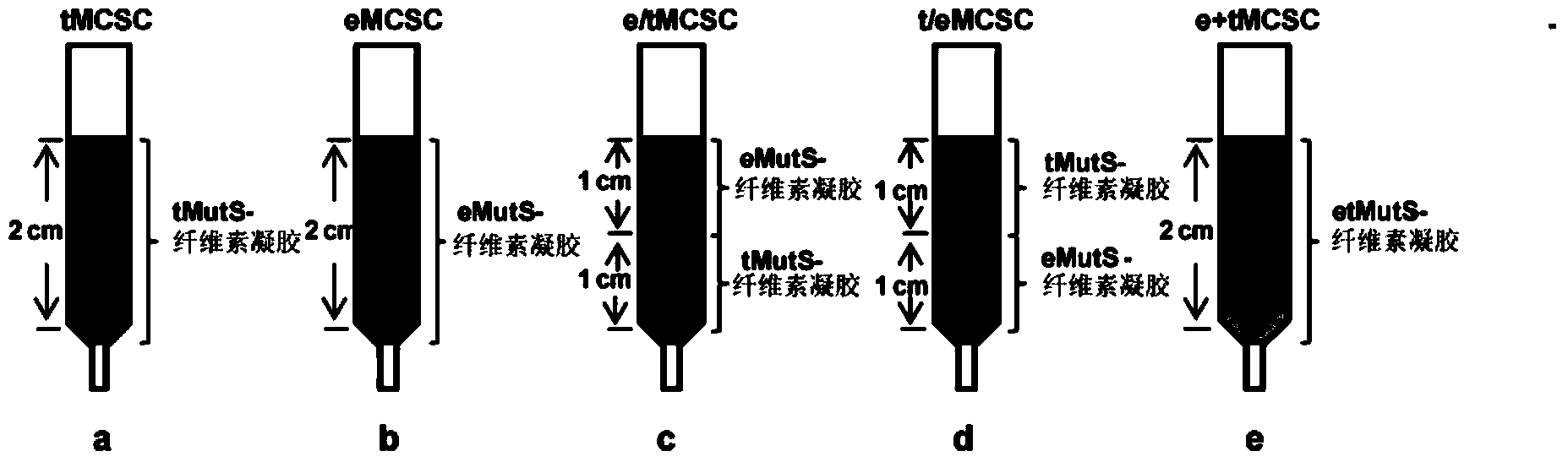
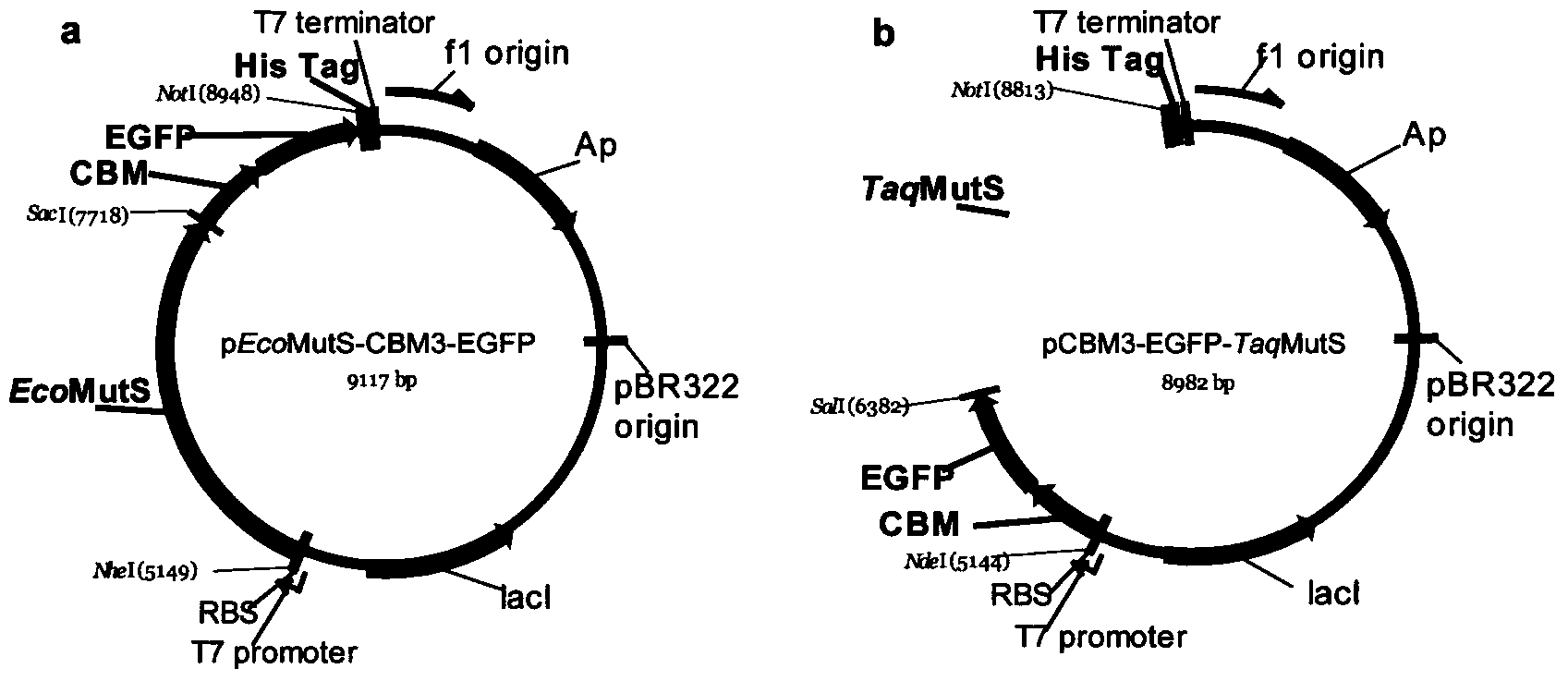
Novel fusion protein of mispairing binding protein and cellulose binding domain 3 and method thereof for removing errors in DNA (Desoxvribose Nucleic Acid) synthesis at high flux
ActiveCN103755815AEffectively fixedLow priceIon-exchange process apparatusOther chemical processesCelluloseCellulose binding
The invention discloses a novel fusion protein of a mispairing binding protein and a cellulose binding domain 3, establishes a method for rapidly immobilizing the fusion protein on cellulose, and a method for constructing a mispairing binding protein cellulose chromatographic column. The invention further establishes a method for removing errors in oligonucleotides synthesized by a chip at high flux by using the mispairing binding protein cellulose chromatographic column. Meanwhile, the method disclosed by the invention is capable of correcting the errors in the DNA synthesis at high flux.
Owner:UNIV OF SCI & TECH OF CHINA
Method for scouring cotton fabric by applying cutinase and CBD (cellulose-binding domain)-alkaline pectinase
InactiveCN102146633AImprove wettabilityImprove qualityBiochemical fibre treatmentVegetal fibresCellulose bindingWoven fabric
The invention provides a method for scouring a cotton fabric by applying cutinase and CBD (cellulose-binding domain)-alkaline pectinase, belonging to the technical field of treatment of cotton fabrics before dyeing and finishing. The method comprises the following steps of: treating a cotton knitted fabric, cotton yarns, or a cotton woven fabric from which size is removed by amylase or sodium hydroxide firstly, in a mixed enzyme solution formed by cutinase and CBD-alkaline pectinase; or respectively treating in the cutinase solution and the CBD-pectinase solution, wherein the hydrophobic surface structure of cotton fibers is destructed by the cutinase, the higher fatty acid ester substances are removed from the surface waxy layers of the cotton fibers, so that the wettability of the cotton fabric is improved, the pectic substances on the cotton fibers are exposed, and then the CBD-pectinase is absorbed on the surface cellulose of the cotton fibers to decompose the pectic substances on the cotton fibers. By the method provided by the invention, on the one hand, the traditional chemical treatment method can be substituted for scouring the cotton fabric, and on the other hand, because the cutinase and the CBD-alkaline pectinase are adopted, the wettability of the cotton fibers and the pectin removal rate are improved.
Owner:JIANGNAN UNIV +1
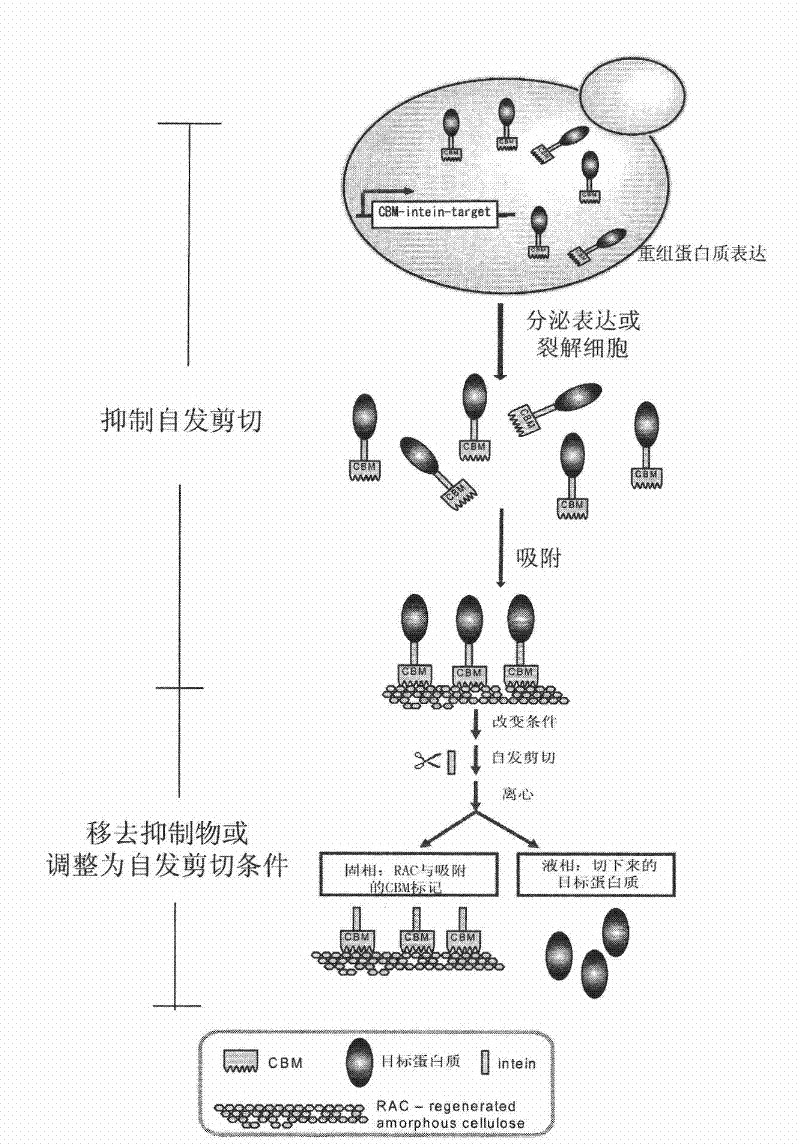
Application of family 3 cellulose binding domain serving as affinity tag for expression and purification of recombinant protein in eukaryote
The invention belongs to the field of recombinant protein expression and purification. The invention provides a method for expressing and purifying recombinant protein in a eukaryote by using a family 3 cellulose binding domain CBM3 serving as an affinity tag for recombinant protein expression. The method comprises the following steps of: (1) constructing a nucleotide sequence of coded CBM3 optimized by a eukaryote codon; (2) constructing a recombinant construct of the nucleotide sequence of the coded CBM3 optimized by the eukaryote codon in the step (1) and a nucleotide sequence of coded target protein; (3) cloning the recombinant construct constructed in the step (2) to an expression vector suitable to be expressed in the eukaryote; (4) expressing the recombinant expression vector obtained in the step (3) in the eukaryote; and (5) purifying the expressed recombinant protein by using the CBM3 as the affinity tag and using cellulose. The invention also provides a method for expressingand purifying the recombinant protein in the eukaryote by using the CBM3 serving as the affinity tag for the recombinant protein expression and internal protein, wherein the method comprises a step of constructing a target protein-internal protein-CBM3 recombinant construct so as to cut out and purify the recombinant protein of fusion expression without additional amino acid.
Owner:UNIV OF SCI & TECH OF CHINA
Enzyme fusion proteins and their use
Cellulase fusion proteins comprising an endoglucanase core region and a heterologous cellulose binding domain are described. The fusion proteins may be produced by recombinant techniques using appropriate polynucleotides, expressing vectors and host cells. The fusion proteins and enzyme preparations thereof are useful in treating cellulosic material, such as textile material, and they are particularly useful in biostoning denim or in biofinishing fabrics and garments. In addition the fusion proteins may be used in pulp and paper industry, oil extraction from plants, detergent compositions, or for improving the quality of animal feed.
Owner:AB ENZYMES OY
Micro-nano lignocellulose, preparation method and applications thereof
ActiveCN110055796ASolve processingSolve concentrationCellulose pulp after-treatment modificationMicro nanoCellulose
The invention provides micro-nano lignocellulose, a preparation method and applications thereof. The micro-nano lignocelluloses contain lignin structures; lignin is bonded with cellulose through hydrogen bonds and chemical bonds. The preparation method comprises following steps: a lignin containing cellulose raw material is dispersed in a hot urea aqueous solution so as to obtain a cellulose raw material dispersion liquid; mechanical pre-treatment is adopted for peeling and grinding so as to obtain a pre-treatment product; and the pre-treatment product is subjected to high pressure homogenizing using a high pressure homogenizer so as to obtain a micro-nano lignocelluloses dispersion liquid. According to the preparation method, removing of the lignin in the lignin containing cellulose raw material is not needed, the hot urea aqueous solution is combined with mechanical pre-treatment and high pressure homogenizing so as to prepare the product; the diameter of the obtained micro-nano lignocelluloses ranges from 5 to 180nm; the length / diameter ratio is 200 or larger; the lignin content ranges from 10 to 35wt%; micro-nano cellulose performance is improved; and the application prospect is promising.
Owner:山东省圣泉生物质石墨烯研究院
Cellulose-binding fibres
InactiveCN1259178AConjugated synthetic polymer artificial filamentsNon-woven fabricsPolyolefinPolymer science
The invention relates to drylaid nonwoven materials comprising bicomponent fibres comprising a low melting polyolefin component and a high melting polyolefin component, the low melting polyolefin component constituting at least a part of the surface of the fibre and comprising a non-grafted polyolefin component and a grafted polyolefin component, wherein the grafted polyolefin component has been grafted with an unsaturated dicarboxylic acid or an anhydride thereof, e.g. with maleic acid or maleic anhydride. The bicomponent fibres fibres have an excellent bonding affinity for natural fibres such as cellulose pulp fibres and allow the production of airlaid nonwovens with reduced generation of dust during the production process and with improved nonwoven strength properties.
Owner:FIBERVISIONS VARDE
Preparation method of flame-retardant Lyocell fiber
InactiveCN109402756AStrong performance close toImprove flame retardant performanceFlame-proof filament manufactureMonocomponent cellulose artificial filamentBreaking strengthCellulose binding
The invention relates to a preparation method of flame-retardant Lyocell fiber. The preparation method is characterized in that flame retardants of tris-(2,3-dibromopropyl)phosphate and cotton pulp are dissolved in an N-methylmorpholine-N-oxide water solution; a spinning solution is prepared; the spinning solution enters a spinning system; sprayed silk threads are vertically stretched in air and enter a coagulating bath tank to be coagulated into fiber; the fiber is subjected to the processes such as alcohol washing, blanching, water washing, oiling and drying; the flame-retardant Lyocell fiber is obtained. The dry breaking strength intensity of the prepared Lyocell fiber is 43 to 45 cN / tex, and the dry breaking elongation is 13.5 to 15 percent; the wet breaking strength of the Lyocell fiber is 31.5 to 33 cN / tex, and the wet breaking elongation of the fiber is 15 to 17 percent; the major strong performance approaches to that of commercially-available Lyocell fiber. The limit oxygen index of the prepared flame-retardant Lyocell fiber reaches 33 percent or higher; after water washing for 20 times, the flame-retardant effect is still not obviously weakened; the result shows that the combining force of the flame retardant and cellulose is high.
Owner:WUHAN TEXTILE UNIV
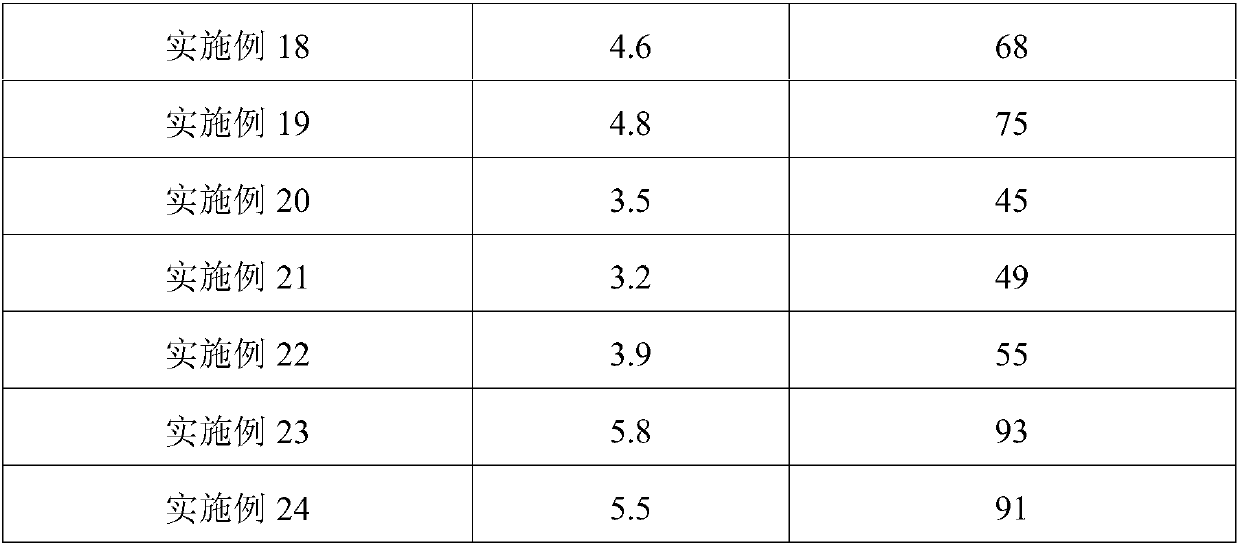
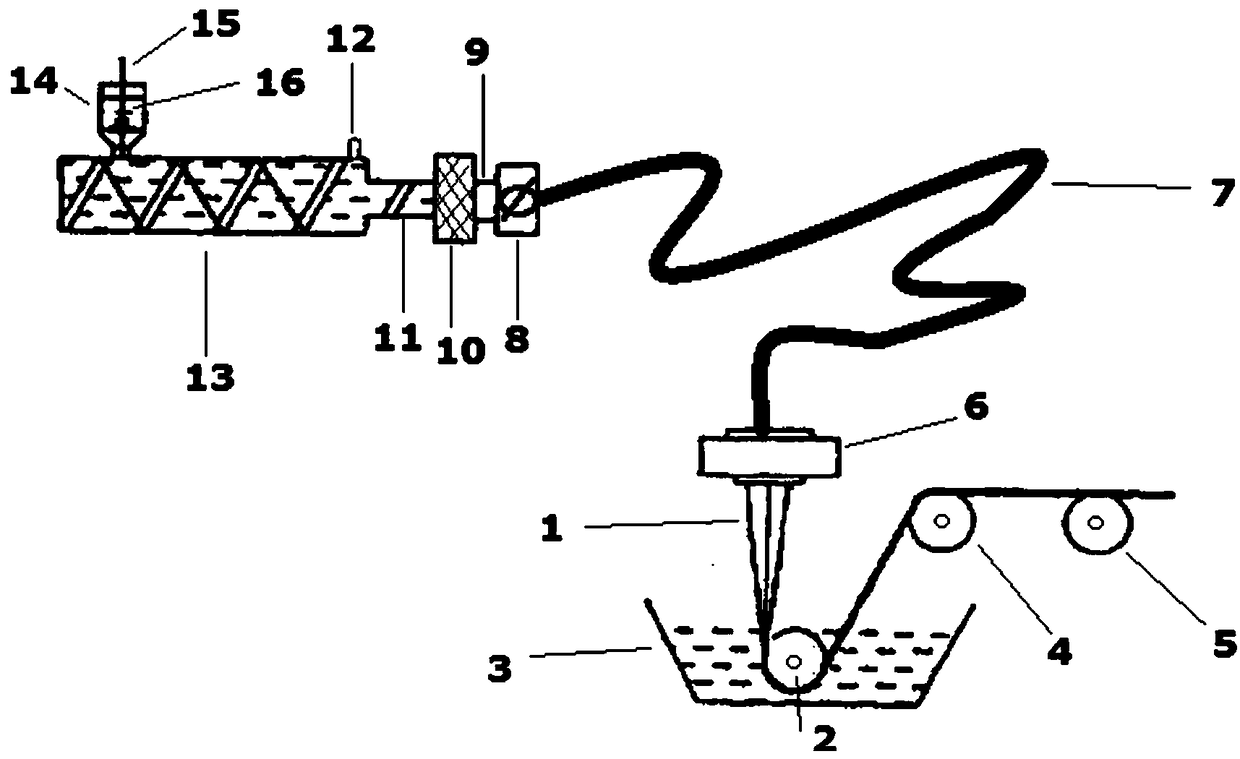
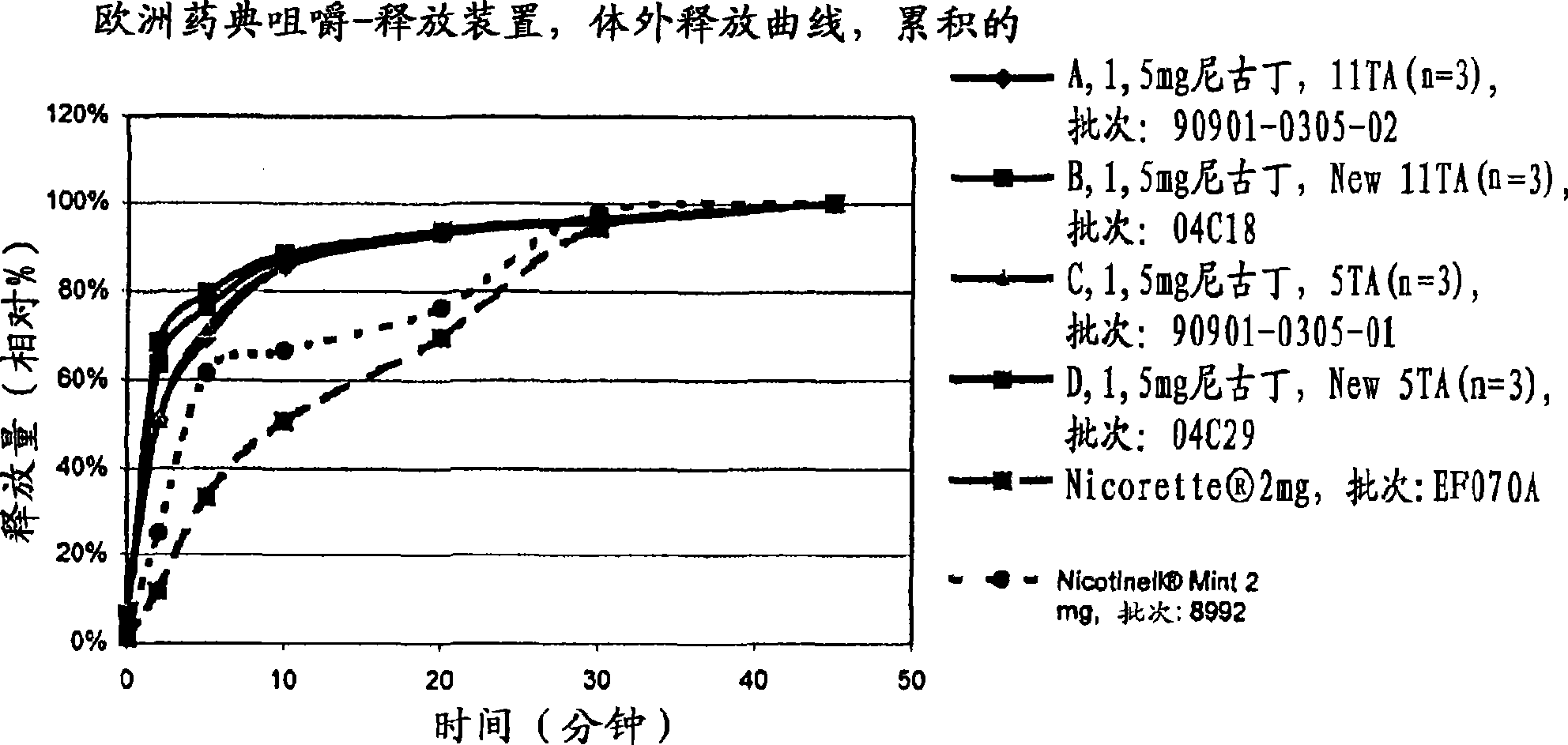
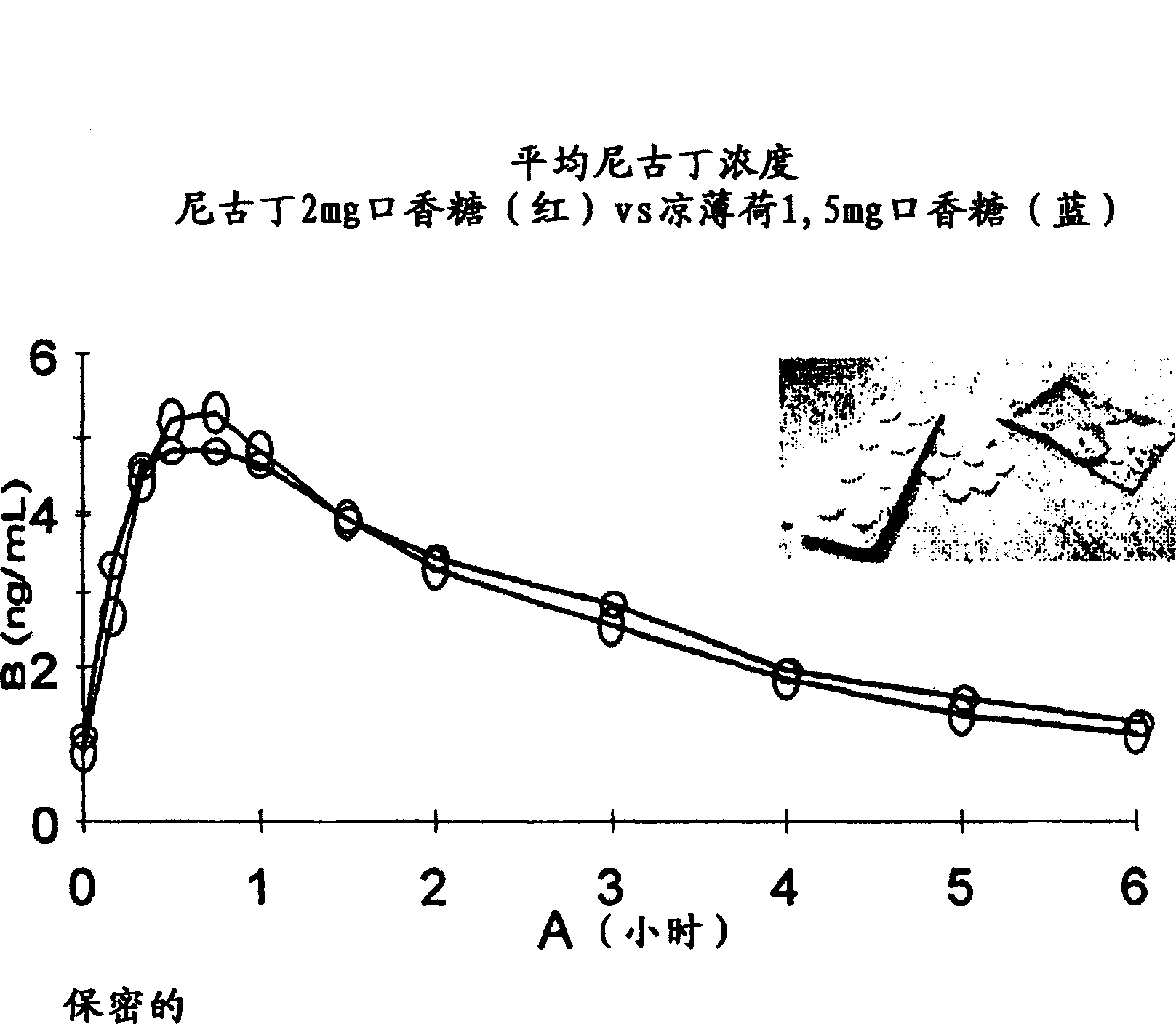
Stable lozenge compositions providing rapid release of nicotine
Use of a nicotine-cellulose combination and a gum base for the preparation of a chewing gum composition for achieving a fast onset of nicotine effect after initiation of chewing the chewing gum composition by a subject. The chewing gum composition is preferably prepared by direct compression and it does not disintegrate during chewing. The invention also relates to chewing gum compositions comprising nicotine, which compositions provide a rapid release of nicotine.
Owner:NICONOVUM AB
Construction, expression and purification method of recombinant human parathyroid hormone in colibacillus
InactiveCN1706947AIncrease productionAvoid the renaturation stepPeptide/protein ingredientsParathyroid hormonesCelluloseEscherichia coli
The present invention relates to medicine biological engineering technology, and is cDNA of small peptide encoding human parathyroid hormone and the method of preparing human parathyroid hormone with its expression vector. Through PCR amplification of chemically synthesized recombinant human human parathyroid hormone (1-34) gene and cloning to expression vector pET-35b(+), recombinant human human parathyroid hormone (1-34) is fused to the carboxyl end of cellulose combining structure domain (CBDclose) and efficient expressed. The fusion protein is treated through cellulose resin affinity chromatographic purification, Factor Xa schizolysis to release rhPTH(1-34), the second cellulose resin affinity chromatography, and C4 reverse efficient liquid chromatographic purification to obtain pure rhPTH(1-34). The sample is tested to have molecular weight of 4117.0 Da.
Owner:南京大学生物制药工程研究中心
Vaccine composition for classical swine fever from plant and manufacturing method thereof
ActiveUS20180305710A1Improve efficiencyImprove securitySsRNA viruses positive-senseViral antigen ingredientsCelluloseAntigen
A recombinant vector for transforming a plant, a plant transformed with the recombinant vector, a plant-made classical swine fever virus antigen pmE2 protein expressed in the plant and uses thereof is provided. By using a recombinant vector having a polynucleotide encoding a GP55 protein of CSFV according to the present invention; and a polynucleotide encoding a cellulose-binding domain protein; and a plant transformed with the recombinant vector, a plant-made classical swine fever virus antigen pmE2 protein may be produced with high efficiency, and has higher safety and stability than those achieved by other production methods. Also, since the plant-derived classical swine fever virus antigen protein pmE2 has a cellulose-binding domain (CBD) protein, it may be usefully used as an effective marker to determine a virus exposure pathway and an antibody producing pathway.
Owner:BIOAPPL +1
Titanium dioxide for decorative paper and preparation method
ActiveCN111334092AGood hueReduced enveloping processInorganic pigment treatmentInorganic compound additionCelluloseCellulose binding
The invention discloses titanium dioxide for decorative paper and a preparation method. The titanium dioxide comprises a titanium dioxide base material and a coating layer located on the surface of the titanium dioxide base material, and the coating layer sequentially comprises a titanium phosphate and aluminum phosphate mixed coating layer and a magnesium oxide and aluminum oxide mixed coating layer from the inside out. According to the titanium dioxide for decorative paper provided by the invention, a titanium phosphate and aluminum phosphate mixed film layer is coated on the surface of titanium dioxide, the hue of the titanium dioxide can be improved, the coating process of the titanium dioxide can be reduced, and the cost is reduced, and then an aluminum oxide and magnesium oxide mixedfilm layer is coated to improve the isoelectric point of the titanium dioxide, so that the titanium dioxide is positively charged in a papermaking system and can be better combined with negatively charged cellulose, the retention rate of the titanium dioxide in paper is improved, and then the covering power of the titanium dioxide is further improved. The invention further provides a preparationmethod of the titanium dioxide by taking the technical scheme as a starting point.
Owner:HENAN BILLIONS NEW MATERIAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com