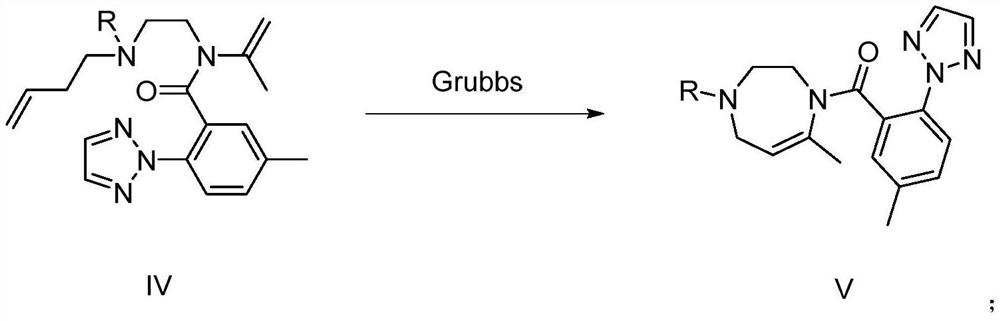
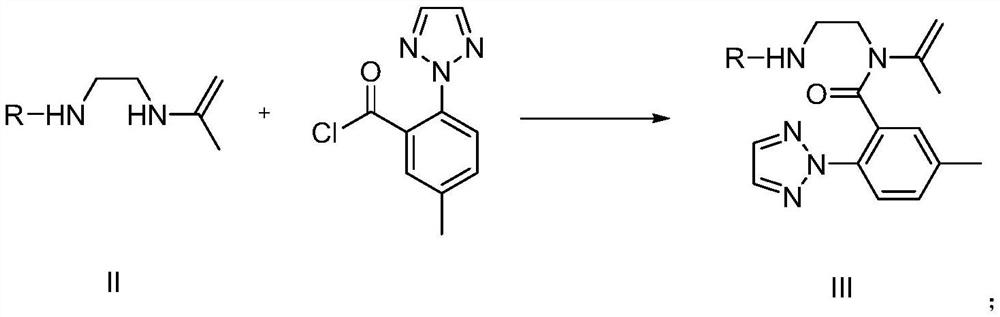
Preparation method and application of suvorexant intermediate
An intermediate and reaction technology, which is applied in the field of preparation of Suwo Leisheng intermediates, can solve the problems of harsh reaction conditions, high risk factor, and difficulty in industrialization, and achieve the effect of mild reaction conditions, high safety factor, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] The preparation of embodiment 1 formula I compound
Embodiment 1-1
[0075]
Embodiment 1-1a
[0077] Add 16.0 g of N-tert-butoxycarbonyl-1,2-ethylenediamine and 14.0 mL of triethylamine into 200 mL of dichloromethane, slowly add 11.8 g of acetyl chloride dropwise, the reaction at room temperature is completed, and TLC until no N-tert Butoxycarbonyl-1,2-ethylenediamine. Water was added to quench the reaction, and the organic phase was separated, washed with water, dried, and filtered to obtain the organic phase, which was concentrated to dryness to obtain 19.1 g of the compound of formula I as a yellow liquid, with a yield of 94.6% and an HPLC purity of 97.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com