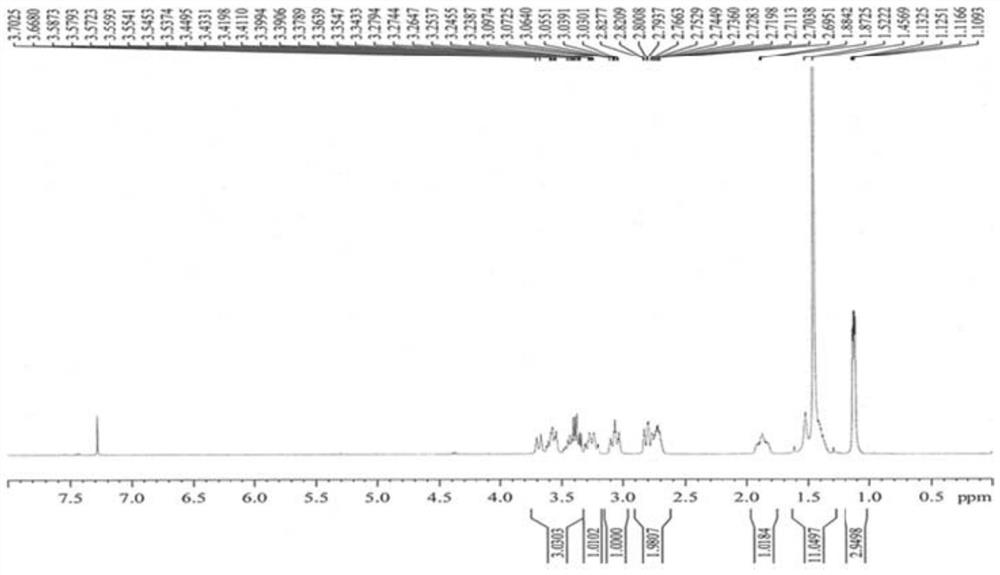
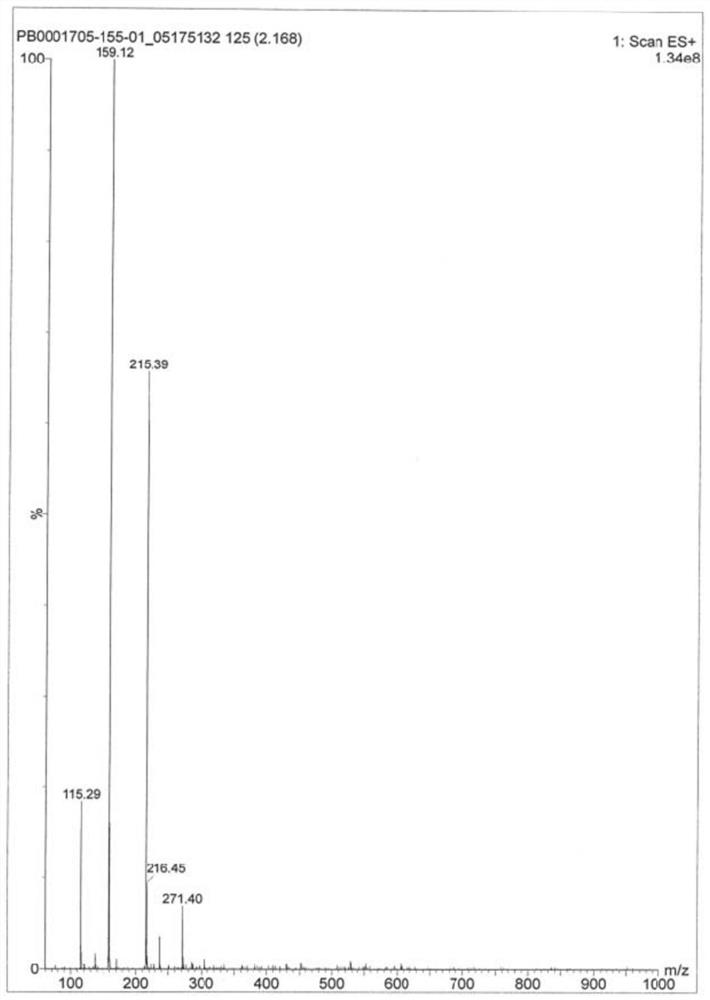
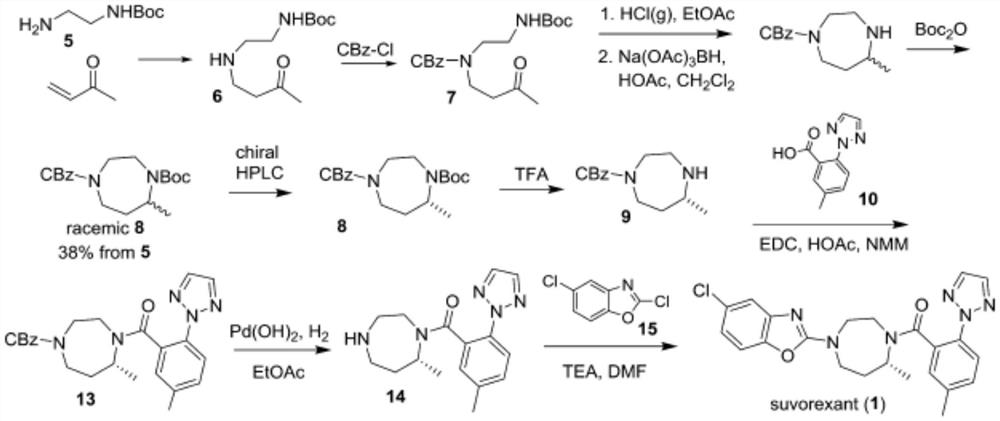
A kind of synthetic method of Suwo Leisheng intermediate
A synthesis method and intermediate technology, applied in the field of pharmaceutical synthesis, can solve the problems of harsh reaction conditions, complex process routes, poor atom economy and the like, and achieve the effects of fewer reaction steps, high reaction yield and high production safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Synthesis of Suvorexan intermediate (5S)-hexahydro-5-methyl-1H-1,4-diazepine-1-carboxylic acid tert-butyl ester
[0048] Step 1: Compound Suvor-2 Synthesis
[0049] Feeding table 1-1
[0050]
[0051] In the reaction kettle, 400 g of solvent tetrahydrofuran and 50 g of raw material compound Suvor-1 were successively added, and the temperature was lowered to -10 to 0 °C. Add 75g of red aluminum solution in batches, control the temperature at -10 ℃ ~ 0 ℃, after the addition, react until the TLC monitoring raw materials disappear, add 200g 10% potassium sodium tartrate solution and 200g ethyl acetate, stir until the system is clarified, and then separate the liquid, Collect the organic phase. Concentrate under reduced pressure to obtain compound Suvor-2, weighing 41.8 g, HPLC purity 98%, yield 88%.
[0052] Step 2: Synthesis of Compound Suvor-3
[0053] Feeding table 1-2
[0054]
[0055] 26.4 g of compound Suvor-2 was dissolved in 132 g of tetrahydrof...
Embodiment 2
[0068] Example 2 Synthesis of Suvor Lexan intermediate (5S)-hexahydro-5-methyl-1H-1,4-diazepine-1-carboxylic acid tert-butyl ester Step 1: Synthesis of compound Suvor-2
[0069] Feeding table 2-1
[0070]
[0071]
[0072] 300 g of solvent tetrahydrofuran and 50 g of raw material compound Suvor-1 were successively added to the reaction kettle, and the temperature was lowered to -10 to 0 °C. Add 50g of red aluminum solution in batches, control the temperature at -10 ℃ ~ 0 ℃, after the addition, react until the TLC monitoring raw materials disappear, add 100g 10% potassium sodium tartrate solution and 100g ethyl acetate, stir until the system is clear, and then separate the liquid, Collect the organic phase. Concentrate under reduced pressure to obtain compound Suvor-2, weighing 40.4 g, HPLC purity 97%, yield 85%.
[0073] Step 2: Synthesis of Compound Suvor-3
[0074] Feeding table 2-2
[0075]
[0076] 26.4 g of compound Suvor-2 was dissolved in 79 g of tetrahydro...
Embodiment 3
[0089] Example 3 Synthesis of (5S)-hexahydro-5-methyl-1H-1,4-diazepine-1-carboxylic acid tert-butyl ester
[0090] Step 1: Compound Suvor-2 Synthesis
[0091] Feeding table 3-1
[0092]
[0093]500 g of solvent tetrahydrofuran and 50 g of raw material compound Suvor-1 were successively added to the reaction kettle, and the temperature was lowered to -10 to 0 °C. Add 100g of red aluminum solution in batches, control the temperature at -10 ℃ ~ 0 ℃, after the addition, react until the TLC monitoring raw materials disappear, add 300g of 10% potassium sodium tartrate solution and 300g of ethyl acetate, stir until the system is clear, and then separate the liquid, Collect the organic phase. Concentrated under reduced pressure to obtain intermediate Suvor-2, weighing 40.6 g, HPLC purity 98%, yield 85%.
[0094] Step 2: Synthesis of Compound Suvor-3
[0095] Feeding table 3-2
[0096]
[0097] 26.4 g of compound Suvor-2 was dissolved in 264 g of tetrahydrofuran, 36 g of gla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com