Synthesis method of anti-insomnia drug Suvorexan intermediate
A synthesis method, methyl technology, applied in the field of synthesis of anti-insomnia drug intermediates, can solve the problems of high cost, expensive raw materials, difficult monitoring, etc., and achieve the effects of easy industrial production, low raw material prices, and low synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] This example relates to a method for synthesizing Suvoraxan intermediate 5-methyl-2-(2H-1,2,3-triazol-2-yl)benzoic acid, which consists of the following steps:
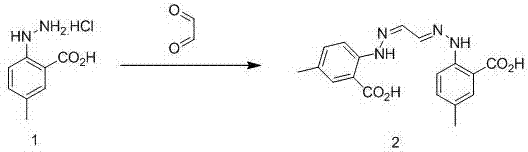
[0026] step one
[0027]
[0028] Add 4-methyl-2-carboxyphenylhydrazine hydrochloride (101.3 g, 0.5 mol) into a 3 L three-necked flask, then add 1 L of 1 mol / L hydrochloric acid solution, stir vigorously until the raw materials are dissolved, and then add dropwise Dialdehyde aqueous solution (mass volume ratio concentration 40%, 36.3g, 0.25mol), control the temperature below 20°C, after the dropwise addition, continue to stir for 2-3 hours, monitor the reaction of raw materials, filter the precipitated solid, 60°C Vacuum drying gave intermediate 2, namely glyoxal di(4′-methyl-2′-carboxy)phenylhydrazone (77.96g, 0.22mol, yield 88%), which can be directly used in the next reaction;
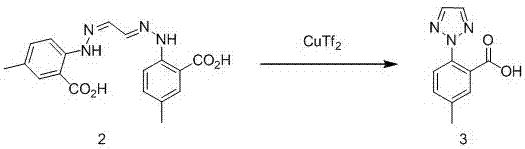
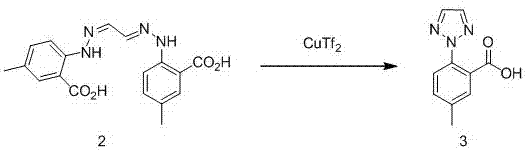
[0029] step two
[0030]
[0031] Add intermediate 2, glyoxal acetal bis(4′-methyl-2′-carboxy)phenylhydrazone (35.4g, 0.1mo...
Embodiment 2
[0033] This example relates to a method for synthesizing Suvoraxan intermediate 5-methyl-2-(2H-1,2,3-triazol-2-yl)benzoic acid, which consists of the following steps:
[0034] step one
[0035]
[0036] Add 4-methyl-2-carboxyphenylhydrazine hydrochloride (101.3 g, 0.5 mol) into a 3 L three-necked flask, then add 1 L of 3 mol / L hydrochloric acid solution, stir vigorously until the raw materials are dissolved, and then add ethyl alcohol dropwise Dialdehyde aqueous solution (mass volume ratio concentration 40%, 46.6g, 0.30mol), control the temperature below 20°C, after the dropwise addition, continue to stir for 2-3 hours, monitor the reaction of raw materials, filter the precipitated solid, 60°C Vacuum drying gave intermediate 2, namely glyoxal di(4′-methyl-2′-carboxy)phenylhydrazone (81.5g, 0.23mol, yield 77%), which can be directly used in the next reaction;
[0037] step two
[0038]
[0039] Add intermediate 2, glyoxal acetal bis(4′-methyl-2′-carboxy)phenylhydrazone ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com