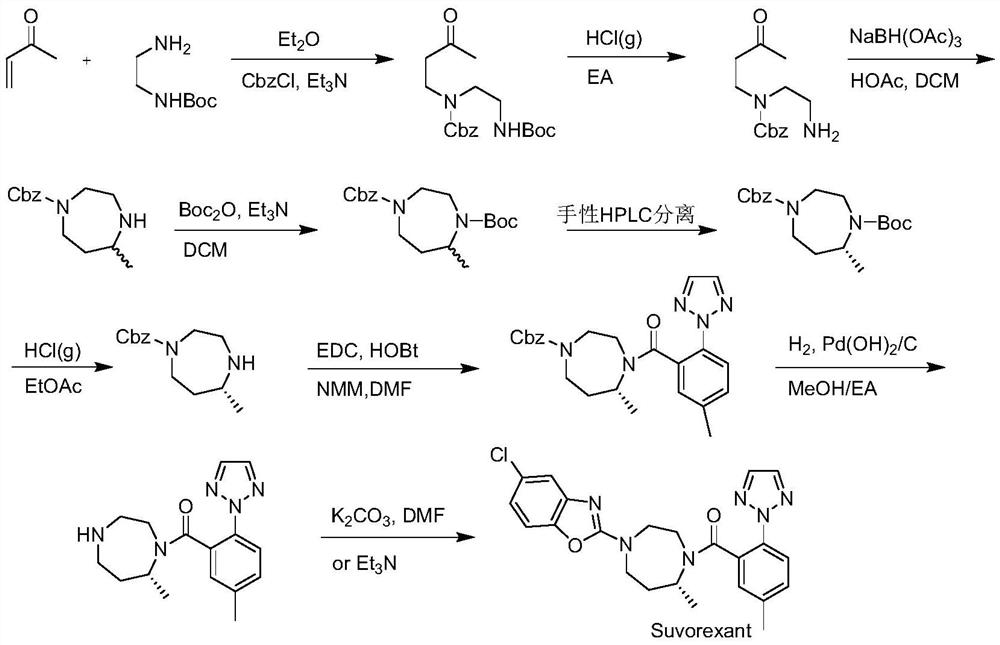
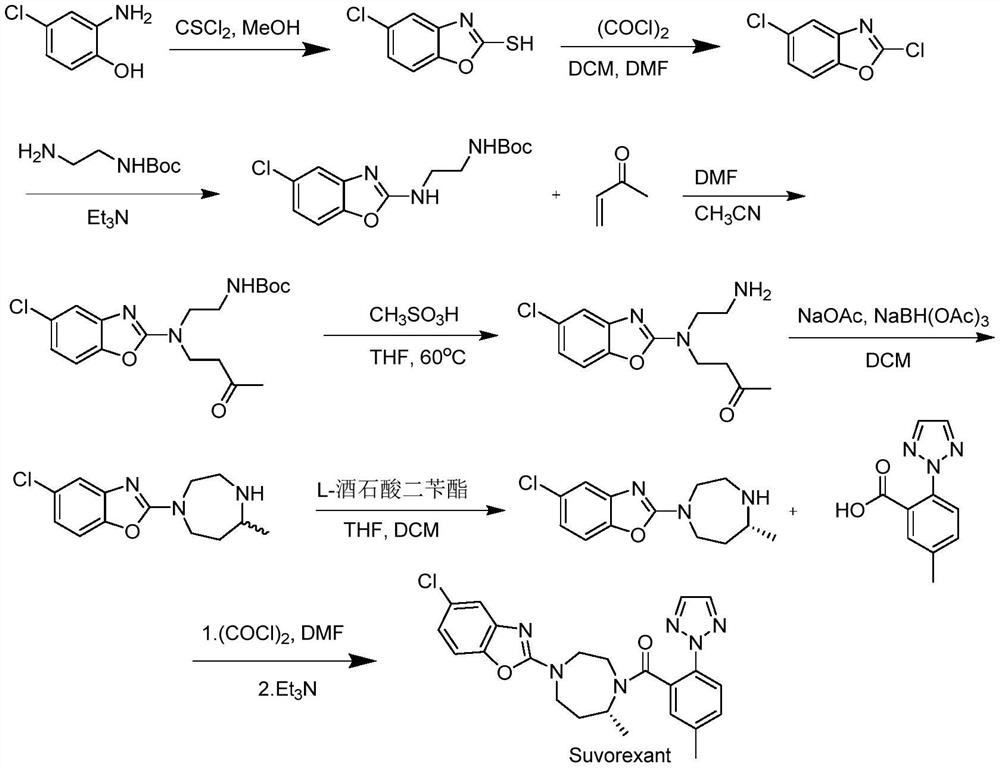
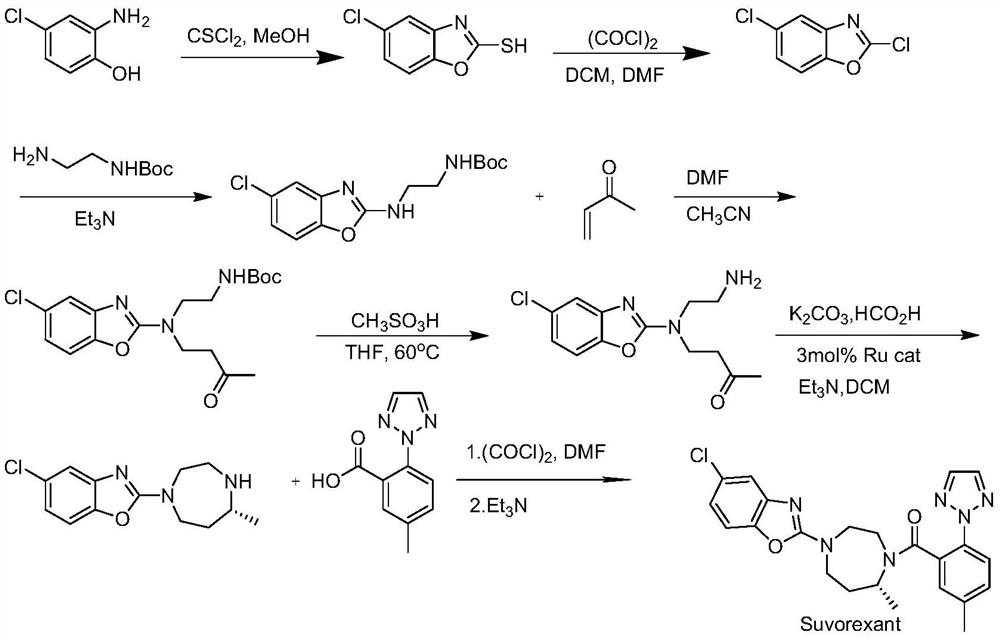
A kind of Suwo Lexan intermediate and preparation method thereof
A technology of compounds and organic solvents, which is applied in the field of synthesizing important intermediates of Suwolexan, can solve the problems of stimulation, expensive enzymes, death, etc., and achieve the effects of easy industrialization, high ee value, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of (S)-methyl 3-(((2-((tert-butoxycarbonyl)amino)ethyl)((R)-1-phenylethyl)amino)butanoate:
[0047]
[0048] Under nitrogen protection, (S)-tert-butyl(2-(((1-phenylethyl)amino)ethyl)carbamate (5.8 g, 22 mmol) was dissolved in 80 mL of THF and cooled to 0 °C. A 1.6M solution of n-butyllithium in hexane was slowly added to the system (30.0 mL, 44 mmol). The resulting solution was stirred for 30 minutes, then cooled to -78 °C, followed by dropwise addition of a solution dissolved in 20 mL of anhydrous Methyl crotonate solution (2.0 g, 20 mmol) in THF. The mixture was stirred at -78° C. for 1 h 30 min. The reaction was quenched after completion and 20 mL of saturated NH was added dropwise 4 Aqueous Cl solution, and the resulting solution was slowly warmed to room temperature. The system was extracted with (2 x 10 mL) EA. The obtained organic phases were combined, dried with anhydrous MgSO4, distilled under reduced pressure and purified to obtain 6.5 g of a co...
Embodiment 2
[0052] Synthesis of (S)-methyl 3-(((2-((tert-butoxycarbonyl)amino)ethyl)((R)-1-phenylethyl)amino)butanoate:
[0053] Under nitrogen protection, (S)-tert-butyl(2-(((1-phenylethyl)amino)ethyl)carbamate (5.8 g, 22 mmol) was dissolved in 80 mL of THF and cooled to 0 C. A 2.0 M solution of lithium diisopropylamide in tetrahydrofuran was slowly added to the system (22.0 mL, 44 mmol), the resulting solution was stirred for 30 minutes, then cooled to -78 C, followed by dropwise addition of a solution dissolved in 20 mL of Methyl crotonate solution (2.0 g, 20 mmol) in water THF. The mixture was stirred at -78° C. for 1 h 30 min. The reaction was quenched after completion and 20 mL of saturated NH was added dropwise 4 Aqueous Cl solution, and the resulting solution was slowly warmed to room temperature. The system was extracted with (2 x 10 mL) EA. The resulting organic phases were combined with anhydrous MgSO 4 After drying, it was distilled under reduced pressure and purified to ob...
Embodiment 3
[0055] Synthesis of (R)-7-methyl-1-((R)-1-phenylethyl)-1,4-diaza-5-lactam:
[0056]
[0057] The Boc protecting group was removed first: the substrate (3.64 g, 10 mmol) was dissolved in 20 mL of DCM, 20 mL of saturated HCl / EA was added, and the reaction was stirred for 6 h. After completion of the reaction the solvent was removed by rotary evaporation and the residue was basified with saturated aqueous NaHCO3 and extracted with DCM. Concentrate the organic phase with anhydrous MgSO 4 dry. A deBoc protected substrate is obtained. The resulting substrate was dissolved in 40 mL of MeOH under N 2 Add CH under protection 3 ONa (0.65 g, 12 mmol) and stirred at room temperature for 12 h. The reaction was cooled to room temperature and washed with saturated NH 4 The Cl aqueous solution was quenched, and then the reaction system was poured into a solution containing 5 wt% Na 2 CO 3 In a separatory funnel of the aqueous solution, after sufficient shaking, it was extracted thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com