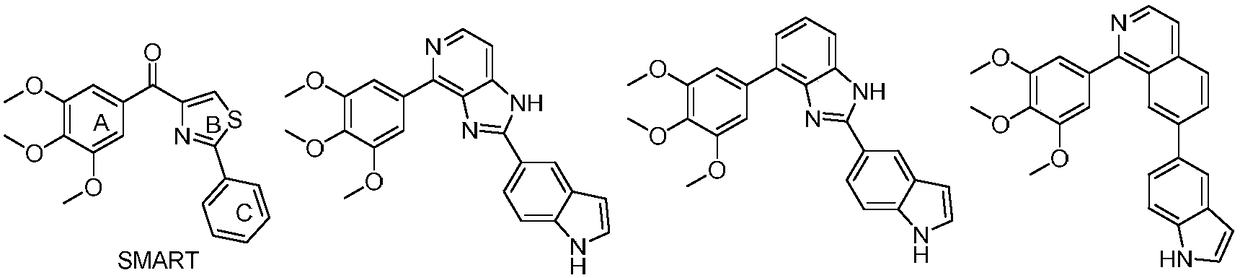
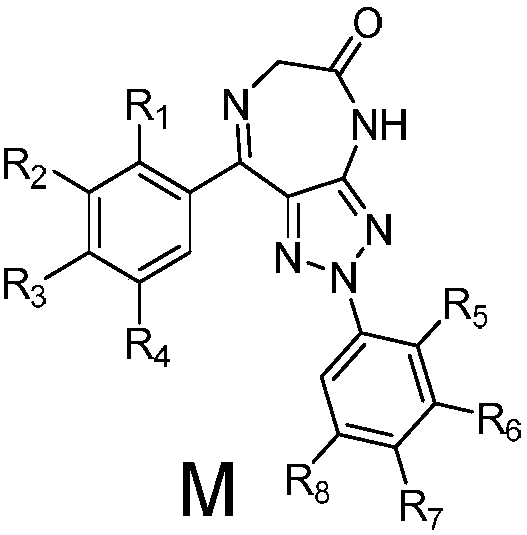
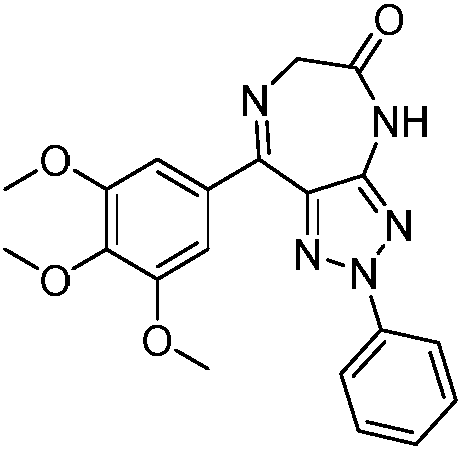
Triazole-diazepine-5-ketone compound
A technology of ketone compounds and diazepines, which is applied in the field of medicine and can solve problems that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1: 2-phenyl-8-(3,4,5-trimethoxyphenyl)-2,6-dihydro-[1,2,3]triazolo[4,5-e][ 1,4] Preparation of diazepin-5(4H)-one (compound 1)
[0098] Add 2-phenyl-4-(3,4,5-benzoyl)-5-amino-1,2,3-triazole (354mg, 1.0mmol) into a 100mL eggplant-shaped bottle, anhydrous di Chloromethane (30mL) dissolved, N 2 Protect, pre-cool at 0°C, add chloroacetyl chloride (0.15 mL, 2.0 mmol) dropwise, and transfer to room temperature after the addition is complete. The reaction was continued for 24 hours. After the reaction was detected by TLC, the solvent was evaporated under reduced pressure, washed with ethyl acetate, and filtered with suction to obtain compound I. Compound 2-chloro-N-(2-phenyl-5-(3,4,5-trimethoxybenzoyl)-2H-1,2,3-triazol-4-yl)acetamide (430mg , 1.0mmol), urotropine (701mg, 5.0mmol), ammonium acetate (386mg, 5.0mmol) was dissolved in 95% ethanol, reflux reaction for 5h, after the reaction was detected by TLC, the solvent was evaporated under reduced pressure, dichlorom...
Embodiment 2
[0099] Example 2: 2-p-tolyl-8-(3,4,5-trimethoxyphenyl)-2,6-dihydro-[1,2,3]triazolo[4,5-e] Preparation of [1,4]diazepin-5(4H)-one (Compound 2)
[0100] Compound 2 was prepared in the same manner as in Example 1 except that the corresponding raw materials were used; the yield was 74%. 1 H NMR (600MHz, CDCl 3 )δ9.61(s,1H),7.96(d,J=8.4Hz,2H),7.31(d,J=8.4Hz,2H),7.30(s,2H),4.59(s,2H),3.92( s,3H),3.89(s,6H),2.42(s,3H)ppm; 13 C NMR (100MHz, CDCl 3 )δ168.0, 162.2, 152.9(×2), 147.1, 140.8, 139.0, 137.0, 134.3, 131.7, 130.1(×2), 118.8(×2), 106.7(×2), 61.0, 57.5, 56.2(×2) ,21.1ppm.
Embodiment 3
[0101] Example 3: 2-o-tolyl-8-(3,4,5-trimethoxyphenyl)-2,6-dihydro-[1,2,3]triazolo[4,5-e] Preparation of [1,4]diazepin-5(4H)-one (compound 3)
[0102] Compound 3 was prepared in the same manner as in Example 1 except that the corresponding raw materials were used; the yield was 76%. 1 H NMR (600MHz, CDCl 3 )δ9.74(s,1H),7.63(m,0.5H),7.55(d,J=7.7Hz,1H),7.43(m,0.5H),7.30(m,1H),7.26(m,1H ),7.18(s,2H),4.51(s,2H),3.82(s,3H),3.79(s,6H),2.39(s,3H)ppm; 13 C NMR (100MHz, CDCl 3)δ167.8, 162.5, 153.0(×2), 146.8, 141.0, 138.7, 134.3, 132.3, 132.0, 129.6, 126.9(×2), 125.0, 106.8(×2), 60.9, 57.2, 56.2(×2), 19.4 ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com