A kind of iridium (Ⅲ) complex and its preparation method and application
A complex and reaction technology, applied in indium organic compounds, chemical instruments and methods, platinum group organic compounds, etc., can solve the problems of poor photostability, concentration quenching, small Stokes shift, etc., and achieve a short synthesis route. , the process is simple, the effect of large Stokes displacement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention provides a preparation method for the iridium (III) complex described in the above technical scheme, comprising the following steps:
[0037] (1) Under anhydrous and oxygen-free conditions, under the action of organic palladium catalysts and basic substances, 2-bromo-4-formic acid ethyl pyridine and phenylboronic acid derivatives are subjected to Suzuki coupling reaction in an organic solvent to obtain 2-Phenylisonicotinic acid ethyl ester derivatives;
[0038] (2) Under anaerobic conditions, carry out the first coordination reaction with the 2-phenylisonicotinic acid ethyl ester derivative obtained in step (1) and iridium trichloride hydrate in a solvent to obtain a bridge chlorine complex; Said solvent is the mixed solvent of organic solvent and water;
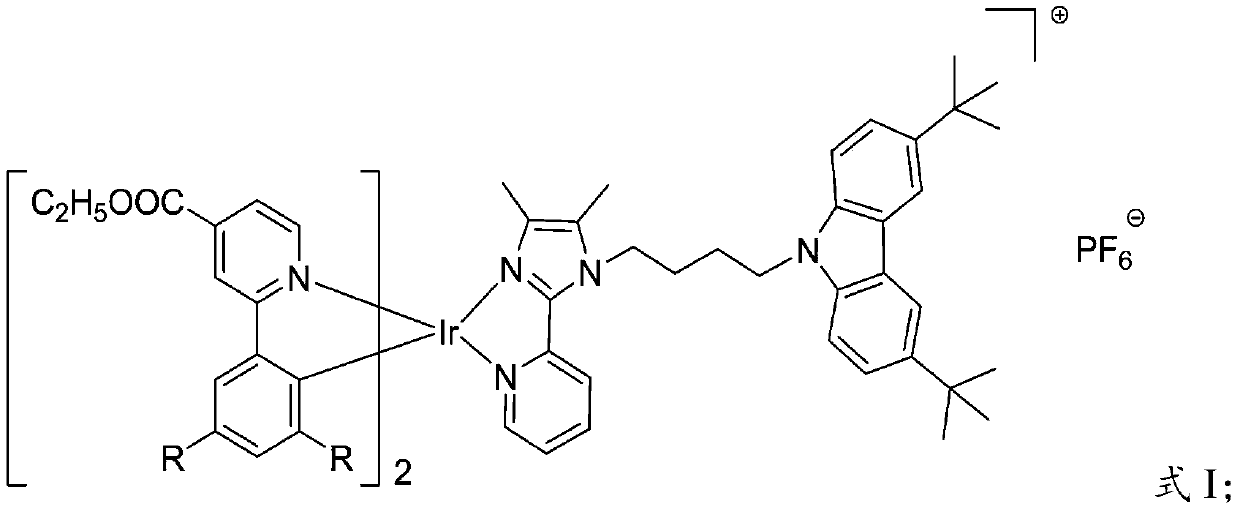
[0039] (3) Under anhydrous and oxygen-free conditions, the bridge chlorine complex obtained in step (2) and 3,6-di-tert-butyl-9-(4-(4,5-dimethyl-2-( Pyridin-2-yl)-1H-imidazol-1-yl)butyl)-9H-car...
Embodiment 1
[0068] Synthesis of Ligand 2-(3,5-Dimethoxyphenyl)isonicotinic acid ethyl ester
[0069]
[0070] In a 100mL two-necked flask, 2.10g of ethyl 2-bromo-4-carboxylate pyridine, 2.01g of 3,5-dimethoxyphenylboronic acid, 4.86g of potassium carbonate, 0.66g of tetrakis(triphenylphosphine)palladium, toluene 60mL, heated at 110°C for 20 hours. After cooling to room temperature, the solution was washed with water (3 x 60 mL). The organic layer was washed with anhydrous MgSO 4 Dry, filter and remove solvent under reduced pressure. Purification by column chromatography (eluent: petroleum ether / ethyl acetate=80:1) gave 1.47 g of white product with a yield of 56%.
[0071] Adopt nuclear magnetic resonance (NMR) to measure the product obtained, the obtained result is: 1 H NMR (400MHz, CDCl 3 )δ8.82(d, J=4.9Hz, 1H), 8.26(s, 1H), 7.79(dd, J=5.0, 1.2Hz, 1H), 7.22(d, J=2.2Hz, 2H), 6.56( t, J=2.2Hz, 1H), 4.45(d, J=7.1Hz, 2H), 3.89(s, 6H), 1.44(t, J=7.1Hz, 3H). 13 C NMR (100MHz, CDCl 3...
Embodiment 2
[0073] Synthesis of Ligand 2-(3,5-Dimethylphenyl)isonicotinic acid ethyl ester
[0074]
[0075] The preparation method is the same as in Example 1, except that 3,5-dimethoxyphenylboronic acid is replaced by 3,5-dimethylphenylboronic acid. A white solid was obtained by column chromatography with a yield of 46%.
[0076] Adopt nuclear magnetic resonance (NMR) to measure the product obtained, the obtained result is: 1 H NMR (400MHz, CDCl 3 )δ8.82(d,J=5.0Hz,1H),8.28(s,1H),7.84–7.74(m,1H),7.67(s,2H),7.10(s,1H),4.45(m,2H ),2.42(s,6H),1.45(dd,J=13.4,6.3Hz,3H). 13 C NMR (100MHz, CDCl 3 )δ165.34, 158.72, 150.24, 138.40, 131.14, 124.85, 120.96, 119.80, 61.82, 21.39, 14.26; test results proved that the obtained product was ethyl 2-(3,5-dimethylphenyl)isonicotinate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com