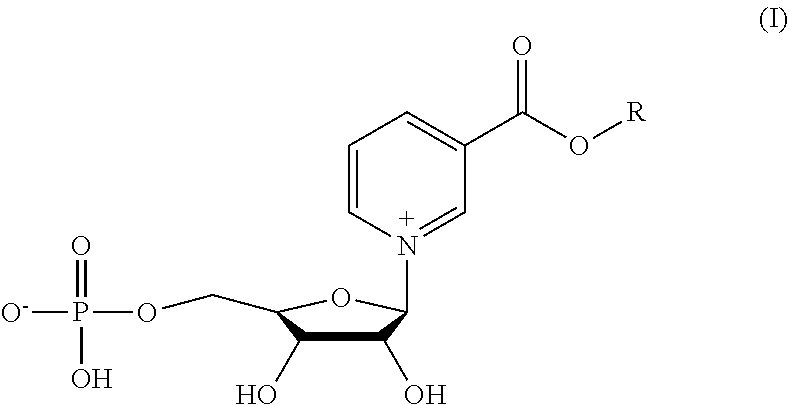
Methyl and ethyl nicotinate-riboside-5-phosphates, preparation thereof and methods of use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0106]This example demonstrates a general synthesis of alkyl (β-nicotinic ribosides.
[0107]Methyl (β-nicotinic riboside and ethyl β-nicotinic riboside were synthesized as described in U.S. Pat. No. 8,106,184, the disclosure of which is incorporated herein by reference.
[0108]General procedure for the synthesis of nicotinate riboside mononucleotides. To a solution of nicotinate riboside in triethyl phosphate was added 2.5 eq. POCl3 at 0° C. The reaction mixture was stirred at 0° C. for 16 h and then neutralized with cold saturated NaHCO3 solution (pH=7). The reaction mixture was concentrated under reduced pressure to minimum volume, and ethyl acetate was added thereto. Filtration afforded pure nicotinate riboside mononucleotide as a solid.
example 2
[0109]This example demonstrates a synthesis of ((2R,3R,4S,5R)-3,4-dihydroxy-5-(3-(ethoxycarbonyl)pyridin-1-ium-1-yl)tetrahydrofuran-2-yl)methyl hydrogen phosphate.
[0110]1-((2R,3S,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-3-(ethoxycarbonyl)pyridin-1-ium trifluoromethanesulfonate (20 mg, 0.085 mm) and triethyl phosphate (1 ml) were placed in a flame-dried round-bottom flask. The mixture was cooled to 0° C., and POCl3 (65 mg, 0.4 mmol) was added dropwise to the mixture. The reaction mixture was stirred at the same temperature for 16 h. After completion, the reaction mixture was neutralized with cold saturated NaHCO3 solution (pH=7). The resulting solution was directly concentrated under reduced pressure to minimum volume, and 2 ml of ethyl acetate was added thereto. Filtration afforded pure compound as a white solid. 1H NMR (500 MHz, D2O): 67 .
example 3
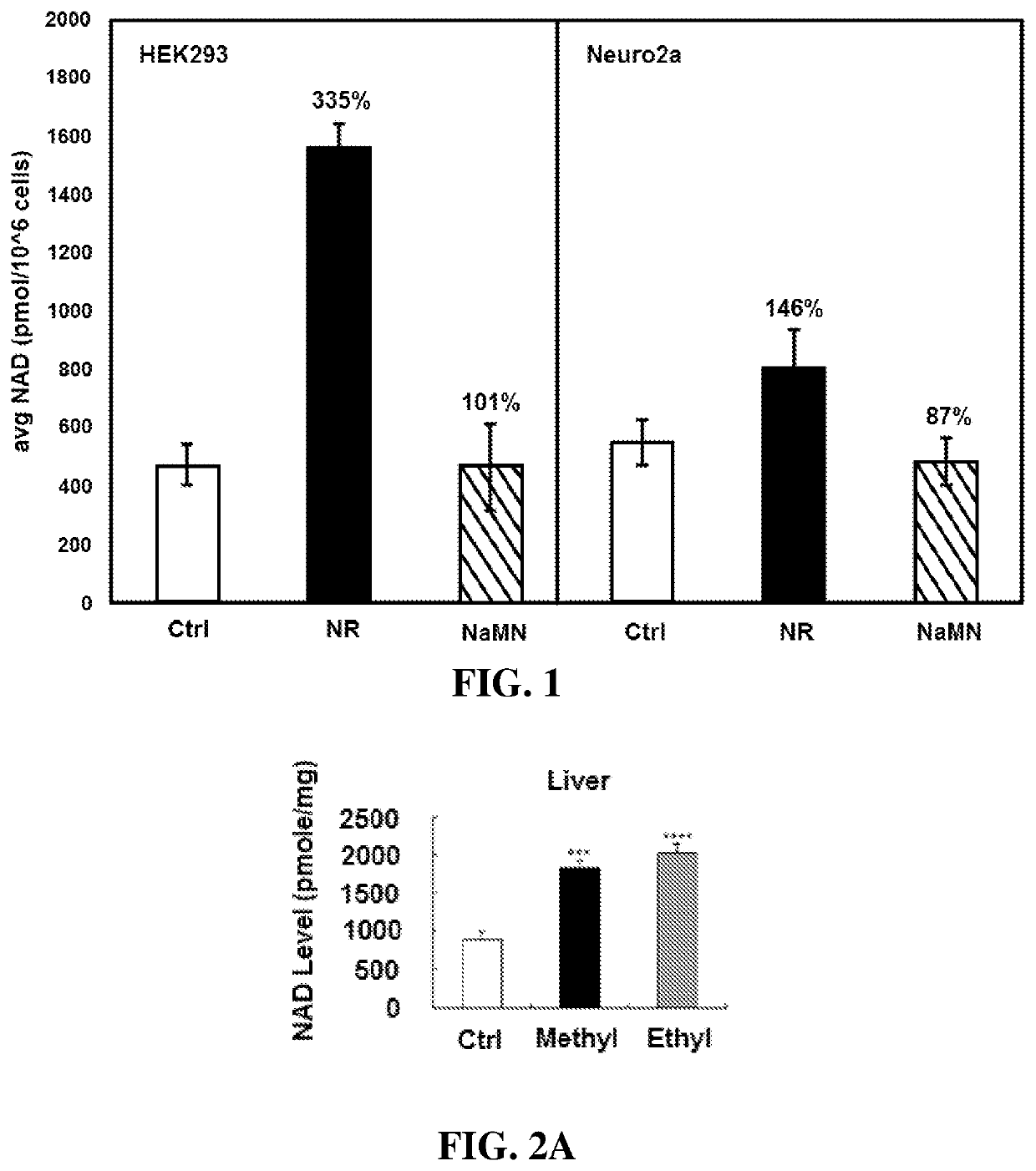
[0111]This example demonstrates the effect of nicotinic acid mononucleotide on intracellular NAD+ levels in HEK293 and Neuro2a cells.
[0112]The ability of nicotinic acid mononucleotide, NaMN, to serve as an NAD+ enhancing agent in mammalian cell lines, Neuro2a and HEK293 cells was examined after an 8 h incubation. NaMN was added to cell growth media at a concentration of 1 mM. All cell treatments were done in duplicate. Nicotinamide riboside (NR) at 1 mM concentration was performed as a positive control. Untreated cells served as negative controls. Cells were treated for the allotted time then harvested by trysin detachment and pelleting. Cells were counted by haemocytometer and then lysed by treatment with 100 μL perchloric acid (7%). Lysates were then neutralized by treatment with NaOH and K2PO4 solutions. NAD+ concentrations were determined by a diaphorase-based assay. NAD+ standards were also run to establish a standard curve. The results are graphically depicted in FIG. 1.
[0113]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com