Synthesis method of cytosine
A synthetic method, cytosine technology, applied in the direction of organic chemistry, etc., can solve the problems that it is difficult to meet the economic benefits of large-scale production, the cost of synthesis conditions is difficult to estimate, and there are great difficulties in operation and promotion, so as to facilitate large-scale application , the yield effect is outstanding, and the effect of reducing the loss of solids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
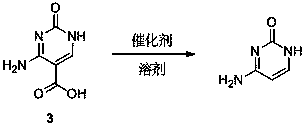
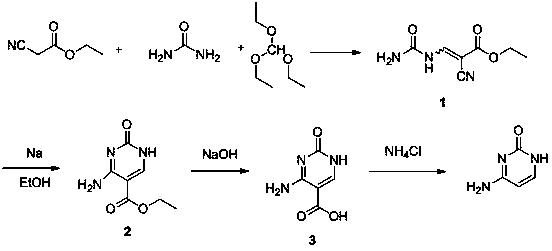
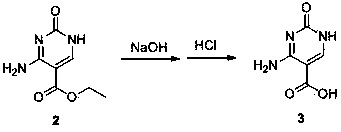
[0041] The synthetic method of cytosine of the present invention comprises the following steps:
[0042] ①Synthesis of ethyl 3-cyano-2-ureidoacrylate: put the reactants ethyl cyanoacetate, urea, and triethyl orthoformate in a molar ratio of 1.0:1.0~2.0:1.0~2.0, put them into the reaction kettle, and stir After uniformity, heat to reflux and keep warm for 2-4 hours. After cooling to room temperature, a solid precipitated, which was collected, washed with a small amount of acetone, and dried;
[0043] ②Synthesis of 5-ethoxycarbonylcytosine: Add the solid obtained above into the reaction kettle, add solvent, alkali and catalyst, stir evenly, heat to 60~100°C, keep it warm for 2 hours, and precipitate the solid after cooling to room temperature, the obtained Dissolve the solid in water with 3 to 8 times the weight of the obtained solid, add acetic acid to acidify to pH = 7, precipitate the solid, collect the solid, wash with a small amount of acetone, and dry;
[0044] The solve...
Embodiment 1
[0053] The specific synthesis method of intermediate 1 is: in a 2500 ml round bottom flask, add 180 grams (3.0 moles) of urea, 370 grams (2.5 moles) of triethyl orthoformate, 226 grams (2.0 moles) of ethyl cyanoacetate, stir Reflux for 4 hours, and constantly observe the phenomenon of reflux to ensure that the reflux is uniform and stable. After stopping the reaction, cool and filter with suction, collect the yellow-white solid, rinse with acetone, and dry to obtain the intermediate 1 , 294.5 grams of yellow-white powdery solid, the yield is 81%.
[0054] Structural characterization: Proton Magnetic Resonance (HNMR) is carried out with a nuclear magnetic resonance instrument, and the relevant signals of atoms under resonance are recorded as follows:
[0055] 1H NMR (CDCl 3 ,400MHz) δ: 1.35 (3H, t, J =7.2, CH 3 ), 4.25 (2H, q, J =7.2, CH 2 O), 7.65 (1H, br s, NH), 7.83 (1H, br s, NH), 8.19 (1H, s, CH), 11.5 (1H, br s, NH).
Embodiment 2
[0057] The specific synthesis method of intermediate 1 is: in a 2500 ml round bottom flask, add 120 grams (2.0 moles) of urea, 256 grams (2.0 moles) of triethyl orthoformate, 226 grams (2.0 moles) of ethyl cyanoacetate, stir Reflux for 4 hours, and constantly observe the phenomenon of reflux to ensure that the reflux is uniform and stable. After stopping the reaction, cool and filter with suction, collect the yellow-white solid, rinse with acetone, and dry to obtain the intermediate 1 , 305.5 grams of yellow-white powdery solid, the yield is 83%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com