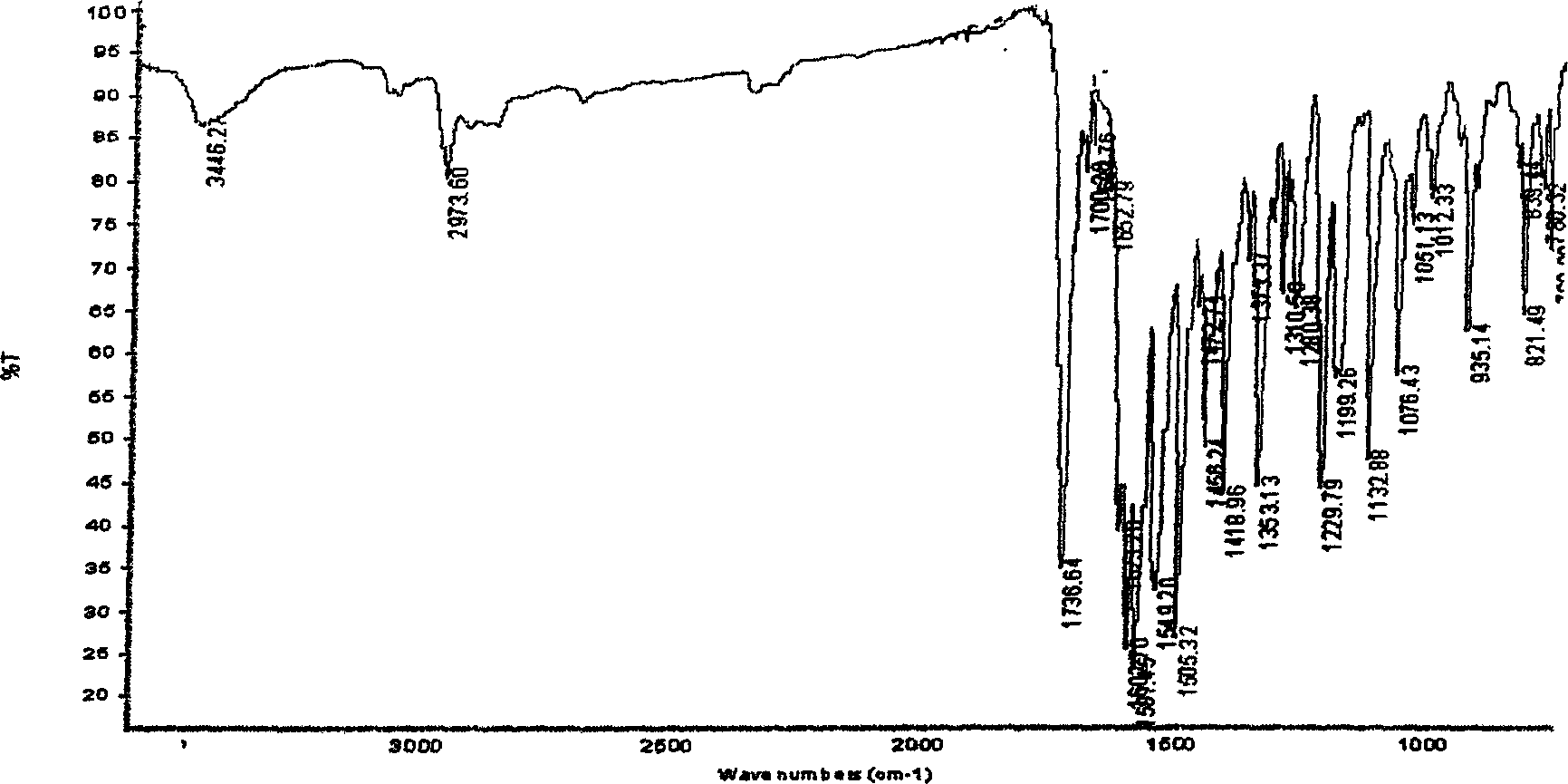
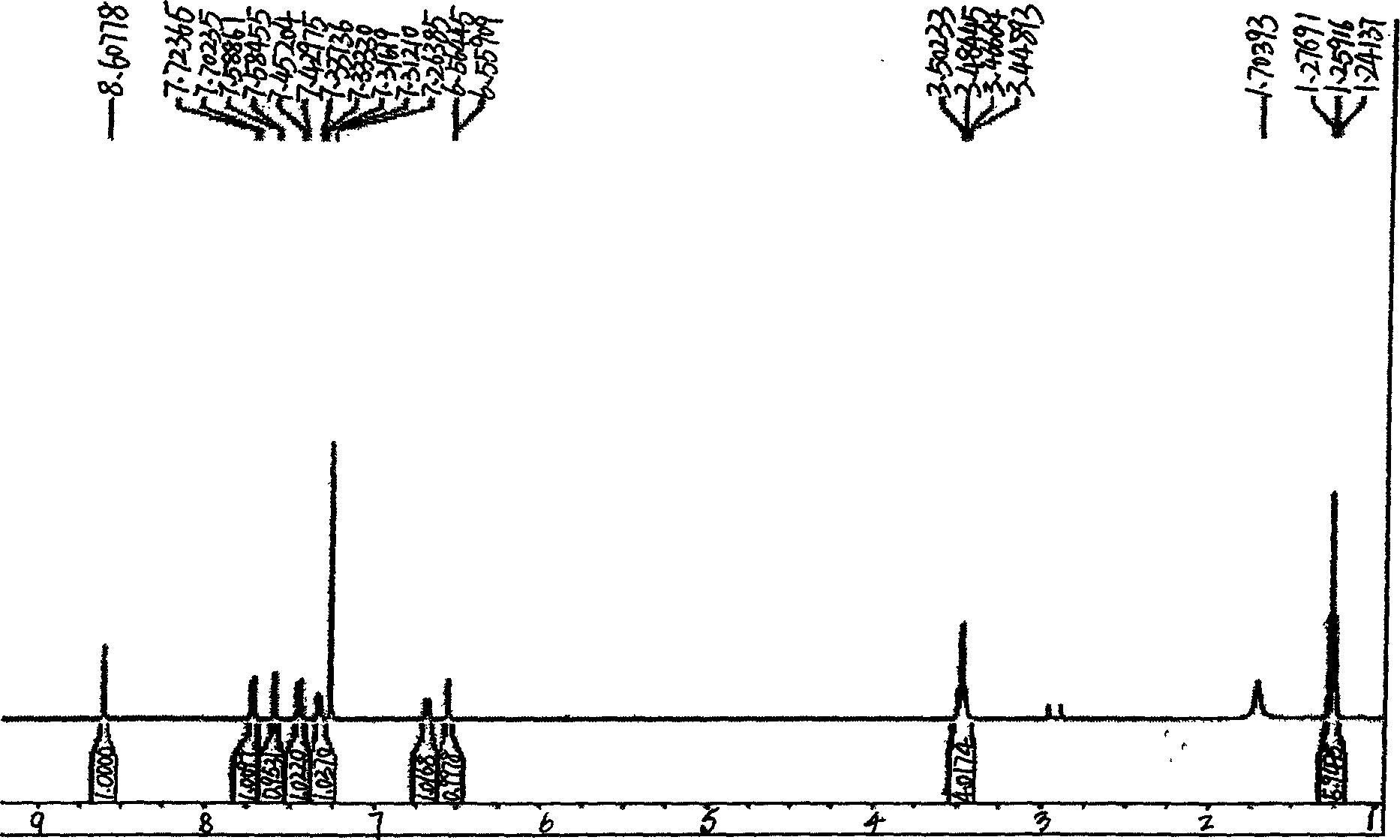
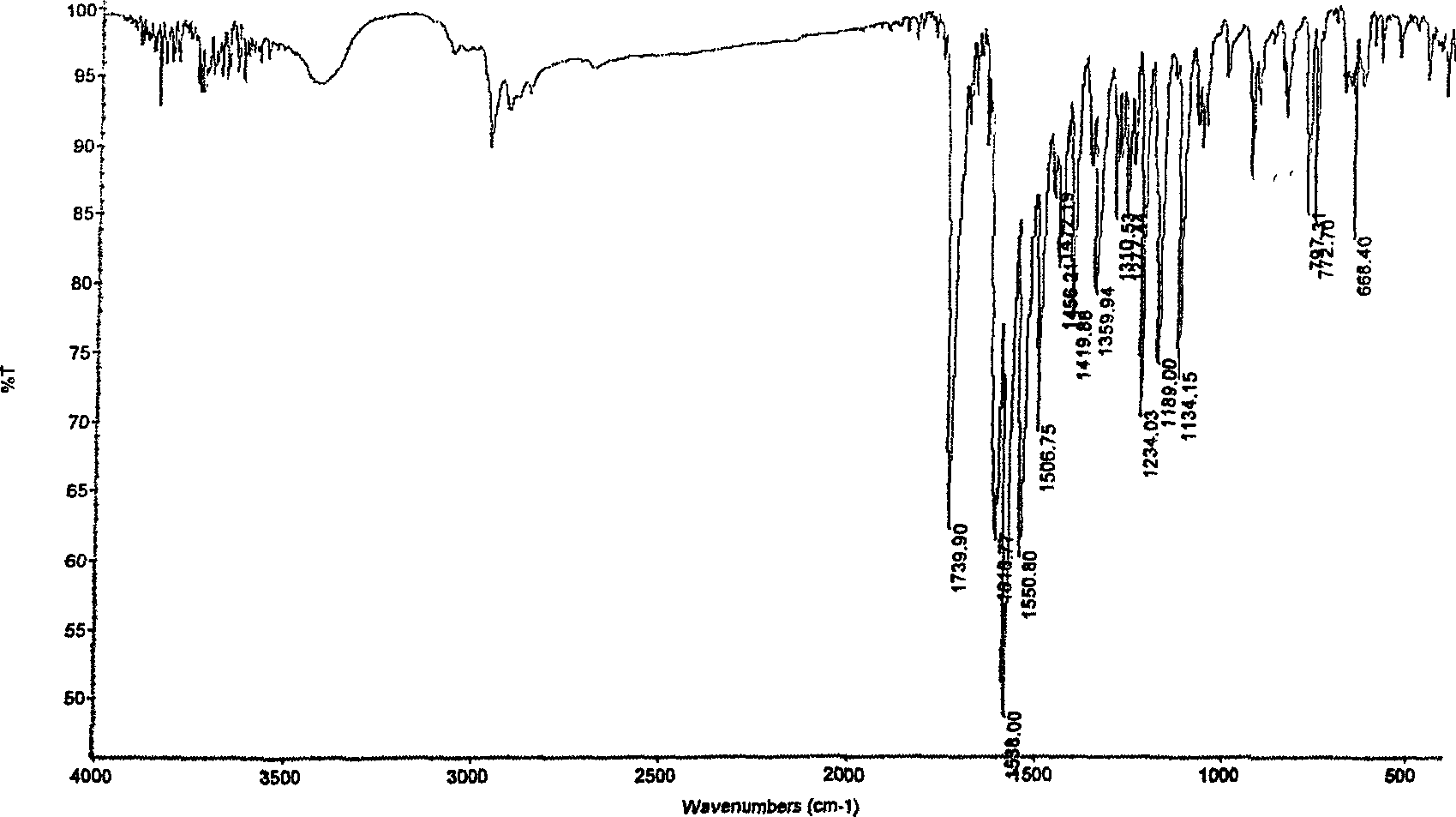
Method for synthesizing 3-(5'-substitution-2-benzoxazole group)-7-diethyl amino group H-1-benzopyrans-2-ketone
A technology of benzoxazolyl and diethylamino, which is applied in the field of synthesis of substituted benzopyrone, can solve the problems of low yield, complicated operation, and many steps, and achieve reduction of reaction steps and simple synthesis process , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Synthesis of 3-(5'-chloro-2-benzoxazolyl)-7-diethylamino-2H-1-benzopyran-2-one
[0043] Add 2g (0.017mol) ethyl cyanoacetate, 3.4g (0.017mol) 4-N, N-diethylaminosalicylaldehyde, 2.5g (0.017mol) 4- Chloro-o-aminophenol, 0.8g (0.0058mol) phenylacetic acid, 50mL n-amyl alcohol, heated to reflux, reacted for 10 hours, evaporated about 3 / 4 of the solvent, cooled to room temperature, added 2% NaOH aqueous solution and stirred for half an hour, filtered After drying, 5.0 g of yellow powder was obtained, the yield: 77.4%, and the melting point was 195-196°C.
Embodiment 2
[0044]Example 2 Synthesis of 3-(5'-methyl-2-benzoxazolyl)-7-diethylamino-2H-1-benzopyran-2-one
[0045] Add 2g (0.017mol) ethyl cyanoacetate, 3.4g (0.017mol) 4-N, N-diethylaminosalicylaldehyde, 2.2g (0.017mol) 4- Methyl o-aminophenol, 1.0g (0.0058mol) p-toluenesulfonic acid, 50mL n-octanol, heated to reflux, reacted for 15 hours, evaporated about 3 / 4 of the solvent, cooled to room temperature, added 2% NaOH aqueous solution and stirred for half hours, filtered and dried to obtain 4.8g of yellow powder, yield: 78%, melting point: 209-210°C.
Embodiment 3
[0046] Example 3 Synthesis of 3-2-benzoxazolyl-7-diethylamino-2H-1-benzopyran-2-one
[0047] Add 2g (0.017mol) ethyl cyanoacetate, 3.4g (0.017mol) 4-N, N-diethylamino salicylaldehyde, 1.9g (0.017mol) o-amino Phenol, 0.9g (0.0058mol) phenylpropionic acid, 50mL butanone, heated to reflux, reacted for 15 hours, evaporated about 3 / 4 of the solvent, cooled to room temperature, added 2% NaOH aqueous solution and stirred for half an hour, filtered and dried, 4.5 g of yellow powder was obtained, the yield: 74.8%, and the melting point was 185-186°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com