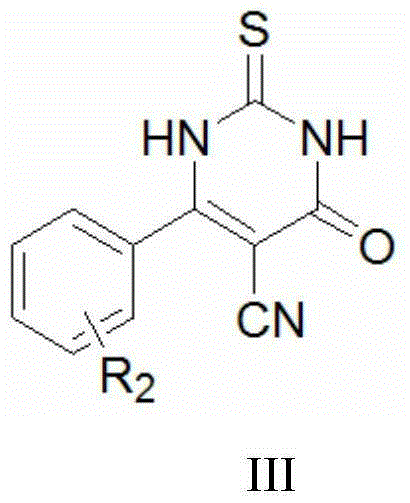
Thiouracil derivatives containing oxadiazole/thiadiazole and preparation method and application of thiouracil derivatives
A technology of thiouracil and its derivatives, which is applied in the field of thiouracil derivatives and their preparation, and can solve the problems of poor antibacterial activity due to drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] According to the concrete synthetic steps of above-mentioned general chemical formula I, when aromatic aldehyde is 2,4-dichlorobenzaldehyde in step (1), i.e. R 2 When being 2,4-dichloro, when the aromatic aldehyde in step (2) is benzaldehyde, ie R 1 When it is hydrogen, the final product compound Ia is obtained according to the above synthesis method, named as: 4-(((5-cyano-4-(2,4-dichlorophenyl)-6-oxo-1,6-dichlorophenyl) Hydropyrimidin-2-yl)thio)methyl)-N-(5-phenyl-1,3,4-oxadiazol-2-yl)benzamide; its chemical structure is:
[0094]
[0095] After detection, compound Ia4-(((5-cyano-4-(2,4-dichlorophenyl)-6-oxo-1,6-dihydropyrimidin-2-yl)thio)methyl) -N-(5-phenyl-1,3,4-oxadiazol-2-yl)benzamide: pale yellow solid, yield 59.6%, m.p.219.2~222.3℃; IR(KBr,cm -1 ):3393(N-H)and1642,1687(2C=O),2364(-CN); 1 HNMR (DMSO-d 6 ,600MHz)δ:8.01(d,J=7.8Hz,2H,ArH),7.76(d,J=7.2Hz,2H,ArH),7.69(d,J=8.4Hz,2H,ArH),7.64(dd ,J=7.2Hz,J=7.8Hz,2H,ArH),7.58(dd,J=7.2Hz,J=7.8Hz,2H,ArH),7.47(d,J=...
Embodiment 2
[0097] According to the concrete synthetic steps of above-mentioned general chemical formula I, when aromatic aldehyde is 2,6-dichlorobenzaldehyde in step (1), i.e. R 2 When being 2,6-dichloro, when the aromatic aldehyde in step (2) is benzaldehyde, ie R 1 When it is hydrogen, the final product compound Ib is obtained, named after: 4-(((5-cyano-4-(2,6-dichlorophenyl)-6-oxo-1,6-dihydropyrimidine-2 -yl)thio)methyl)-N-(5-phenyl-1,3,4-oxadiazol-2-yl)benzamide.
[0098] After detection, compound Ib4-(((5-cyano-4-(2,6-dichlorophenyl)-6-oxo-1,6-dihydropyrimidin-2-yl)thio)methyl) -N-(5-phenyl-1,3,4-oxadiazol-2-yl)benzamide: light yellow solid, yield 56.1%, m.p.204.4~206.1℃; IR(KBr,cm -1 ):3407(N-H)and1632,1682(2C=O),2361(-CN); 1 HNMR (DMSO-d 6 ,600MHz)δ:7.96(d,J=7.8Hz,2H,ArH),7.78(d,J=7.2Hz,2H,ArH),7.66(d,J=8.4Hz,2H,ArH),7.60(dd ,J=7.2Hz,J=7.8Hz,2H,ArH),7.53(dd,J=7.2Hz,J=7.8Hz,2H,ArH),7.44(d,J=3.6Hz,1H,ArH),7.35 (d,J=6.0Hz,1H,ArH),4.40(s,2H,CH 2 ); 13 CNMR (CD 3 OD,150MHz)δ:3...
Embodiment 3
[0100] According to the specific synthetic steps of above-mentioned general chemical formula I, when aromatic aldehyde is p-phenylbenzaldehyde in step (1), i.e. R 2 When being 4-phenyl, when the aromatic aldehyde in step (2) is benzaldehyde, ie R 1 When it is hydrogen, the final product compound Ic is obtained, named as: 4-(((5-cyano-4-(1,1'-biphenyl-4-yl)-6-oxo-1,6-di Hydropyrimidin-2-yl)thio)methyl)-N-(5-phenyl-1,3,4-oxadiazol-2-yl)benzamide.
[0101] After detection, compound Ic4-(((5-cyano-4-(1,1'-biphenyl-4-yl)-6-oxo-1,6-dihydropyrimidin-2-yl)thio )methyl)-N-(5-phenyl-1,3,4-oxadiazol-2-yl)benzamide: light yellow solid, yield 53.6%, m.p.260.2~262.8℃; IR(KBr, cm -1 ):3409(N-H)and1632,1689(2C=O),2361(-CN); 1 HNMR (DMSO-d 6 ,600MHz)δ:8.33(d,J=7.2Hz,2H,ArH),8.02(d,J=8.4Hz,2H,ArH),7.89(d,J=8.4Hz,2H,ArH),7.77(d ,J=7.8Hz,2H,ArH),7.74(d,J=7.8Hz,2H,ArH),7.71(d,J=8.4Hz,2H,ArH),7.64(dd,J=7.2Hz,J= 7.8Hz, 2H, ArH), 7.59(dd, J=7.2Hz, J=7.8Hz, 1H, ArH), 7.50(dd, J=7.2Hz, J=8.4Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com