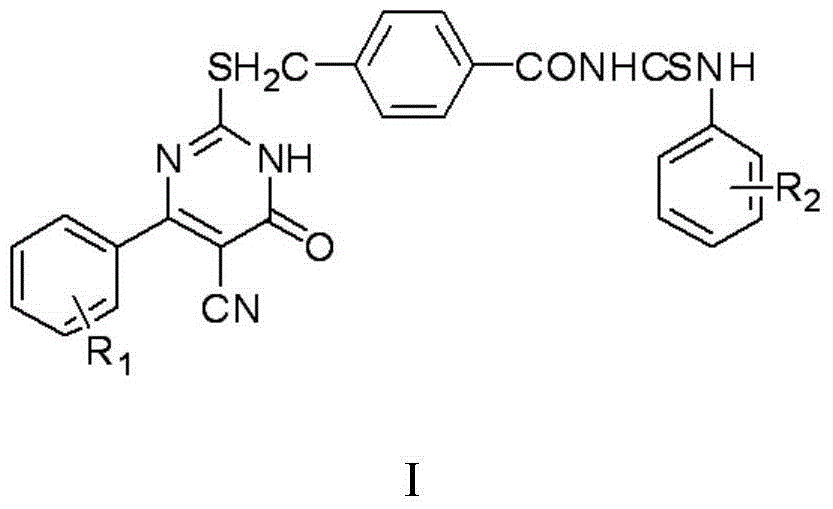
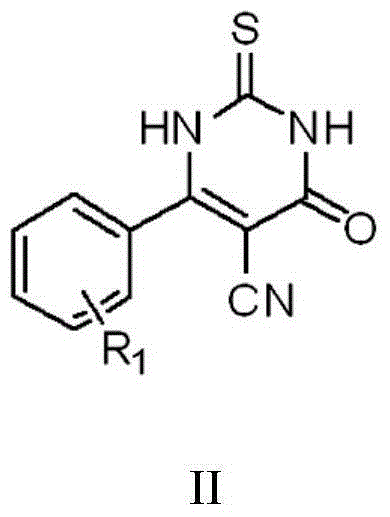
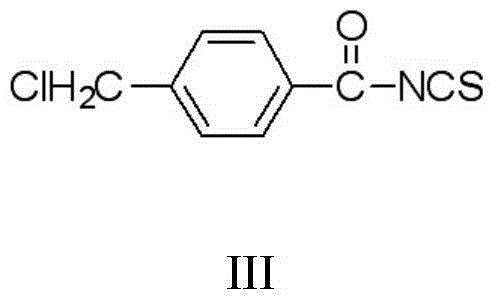
Thiouracil derivative, preparation method and application thereof
A technology of thiouracil and derivatives is applied in the field of thiouracil derivatives and their preparation, and achieves the effects of simple operation, strong antibacterial activity and widening of selection range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Add 10mmol aromatic aldehyde, 10mmol ethyl cyanoacetate, and 10mmol thiourea to a 250mL flask, add 100mL absolute ethanol to dissolve, add 2mL piperidine as a catalyst, and reflux at 92°C for 12 hours; cool to room temperature , produce a lot of solids, filter to get the crude product, dissolve the crude product in 0.5mmol / L NaOH solution, wash three times with ethyl acetate, collect the water phase in a beaker, adjust the pH value to 3 with 1mmol / L HCl solution Left and right, separate out a large amount of solids, filter, dry, obtain intermediate compound II; Its chemical reaction process is:
[0044]
[0045] where R 1 is hydrogen, halogen or aromatic, preferably one of hydrogen, chlorine, bromine, fluorine or phenyl;
[0046](2) Add 12mmol potassium thiocyanate (KSCN) and 25mL water into a 250mL flask, stir to dissolve KSCN, then add 20mL toluene and 1mmol tetrabutylammonium bromide (TBAB), stir electromagnetically at room temperature for 30 minutes, and dis...
Embodiment 2
[0056] When the aromatic aldehyde is benzaldehyde, ie R 1 For hydrogen, the arylamine is 4-chloroaniline, ie R 2 For chlorine, the final product compound Ib is obtained according to the preparation method of Example 1, named after: 4-[(5-cyano-6-oxo-4-phenyl-1,6-dihydropyrimidin-2-ylsulfur Substitute) methyl]-N-(4-chlorophenyl)benzoylthiourea;
[0057] After detection, compound Ib (4-[(5-cyano-6-oxo-4-phenyl-1,6-dihydropyrimidin-2-ylthio)methyl]-N-(4-chlorobenzene Base) Benzoylthiourea): Pale yellow solid, yield 65.4%, m.p.186.2~187.3℃; 1 H NMR (CD 3 OD,600MHz)δ:7.92(d,J=8.4Hz,2H,ArH),7.74(d,J=7.8Hz,2H,ArH),7.66(d,J=7.8Hz,2H,ArH),7.62( d,J=1.8Hz,1H,ArH),7.48(d,J=7.2Hz,1H,ArH),7.42(m,J=4.2Hz,3H,ArH),7.28(t,J=8.4Hz,1H ,ArH),4.36(s,2H,CH 2 ); 13 C NMR (CD 3 OD,150MHz)δ:36.7,93.4,115.6,126.4,126.4,127.5,127.5,127.8,127.8,127.9,129.1,129.1,131.1,131.1,131.6,133.7,136.6,136.8,160.8,1605.9 180.2; IR (KBr) v: 3396 (N-H) cm -1 ,1650(C=O)cm -1 ,1540(N-H,C-N)cm -1 ,1309(C-N,N...
Embodiment 3
[0059] When the aromatic aldehyde is benzaldehyde, ie R 1 is hydrogen, and the arylamine is 2-methylaniline, namely R 2 For methyl, the final product compound Ic is obtained according to the preparation method of Example 1, named as: 4-[(5-cyano-6-oxo-4-phenyl-1,6-dihydropyrimidin-2-yl Thio)methyl]-N-(2-methylphenyl)benzoylthiourea;
[0060] After detection, compound Ic (4-[(5-cyano-6-oxo-4-phenyl-1,6-dihydropyrimidin-2-ylthio)methyl]-N-(2-methyl Phenyl)benzoylthiourea): pale yellow solid, yield 63.5%, m.p.185.0~188.3℃; 1 H NMR (CD 3 OD,600MHz)δ:7.95(d,J=7.8Hz,2H,ArH),7.79(d,J=8.4Hz,2H,ArH),7.62(d,J=7.2Hz,1H,ArH),7.55( d,J=4.2Hz,4H,ArH),7.42(d,J=6.6Hz,1H,ArH),7.30(d,J=6.6Hz,1H,ArH),7.24(m,J=4.8Hz,2H ,ArH),4.38(s,2H,CH 2 ),2.28(s,3H,CH 3 ); 13 C NMR (CD 3 OD, 150MHz) δ: 17.9, 36.7, 93.4, 115.8, 126.0, 126.4, 126.4, 127.5, 127.5, 127.8, 127.8, 128.6, 128.6, 129.7, 130.8, 131.6, 135.9, 139.4, 160.8, 160.5, 160.5, 4, 180.3; IR (KBr) v: 3444 (N-H) cm -1 ,1650(C=O)cm -1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com