2-amino-6-chloropurine as well as synthesis method, intermediate and application thereof
A synthetic method, the technology of chloropurine, which is applied in the field of medicine, can solve the problems of high equipment requirements, viscous materials, difficulty in suction filtration/centrifugation, etc., and achieve the effect of cheap and easy-to-obtain raw materials, few reaction steps, and good industrial prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
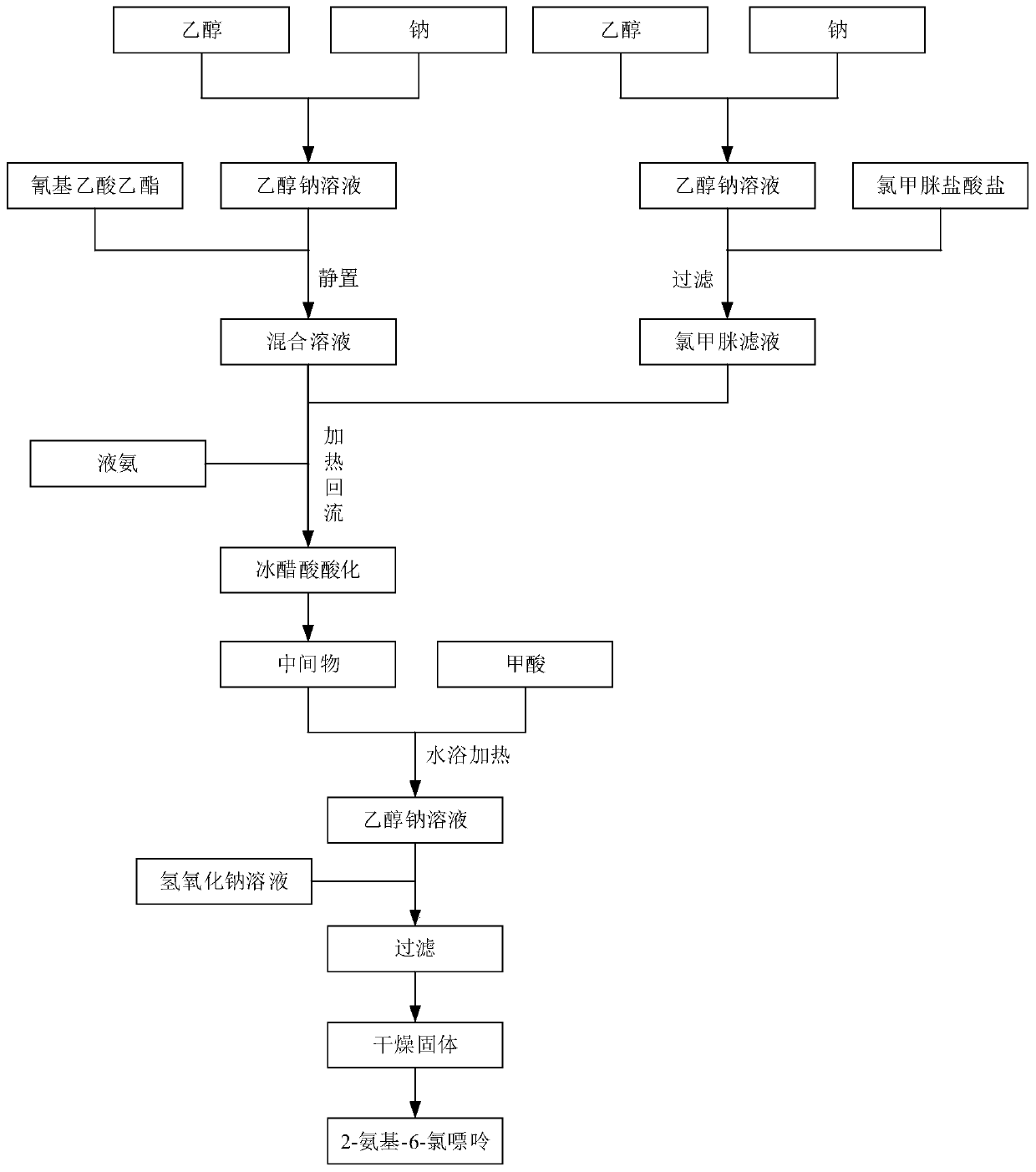
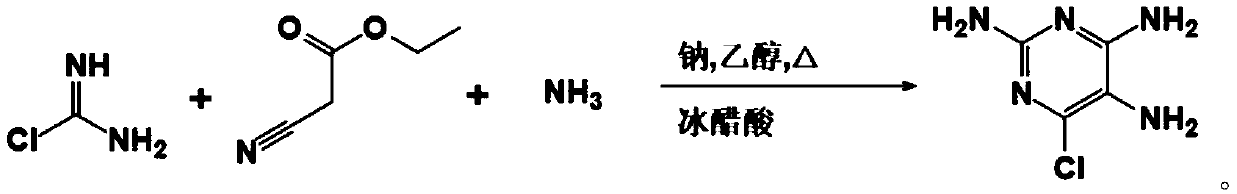
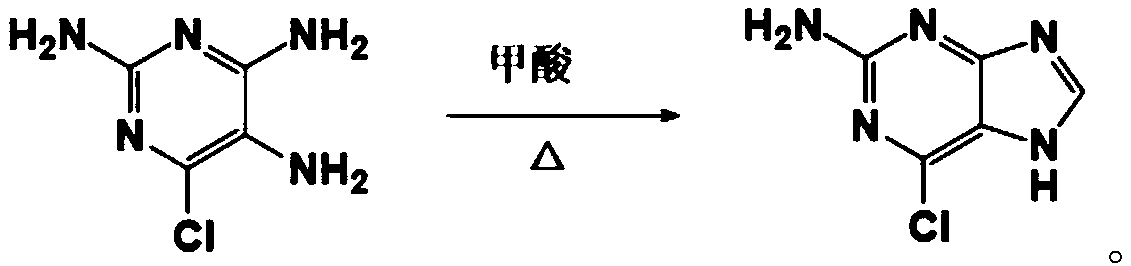
[0032] Such as figure 1 As shown, the present embodiment 1 provides a kind of synthetic method of 2-amino-6-chloropurine, comprises the following steps: step S1, one reaction, i.e. ethyl cyanoacetate, chloroformamidine hydrochloride, liquid ammonia and Sodium mixed reaction to generate intermediate; step S2, secondary reaction, that is, intermediate and formic acid mixed reaction; step S3, after cooling, slowly add 10% sodium hydroxide solution to the product of the secondary reaction, and thoroughly Mix until the mixture is just alkaline with litmus; and step S4, filter with a Buchner funnel, collect the solid by suction, and then dry to obtain the 2-amino-6-chloropurine.
[0033] Optionally, the molar ratio of ethyl cyanoacetate, chloroformamidine hydrochloride, liquid ammonia, sodium, and formic acid is 1:1.1-1.2:2-3:0.1:1-1.5.
[0034] The synthesis method of the present embodiment 1 selects ethyl cyanoacetate, chloroformamidine hydrochloride, and liquid ammonia as raw ma...
Embodiment 2
[0047] On the basis of Example 1, this Example 2 provides a kind of 2-amino-6-chloropurine, its structural formula is:
[0048]
[0049] For the specific structure and implementation process of 2-amino-6-chloropurine, please refer to the relevant discussion in Example 1, and details will not be repeated here.
Embodiment 3
[0051]On the basis of Example 1, this Example 3 provides an intermediate for synthesizing 2-amino-6-chloropurine, whose structural formula is:
[0052]
[0053] For the specific structure and implementation process of the intermediate, please refer to the related discussion of Embodiment 1, which will not be repeated here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com