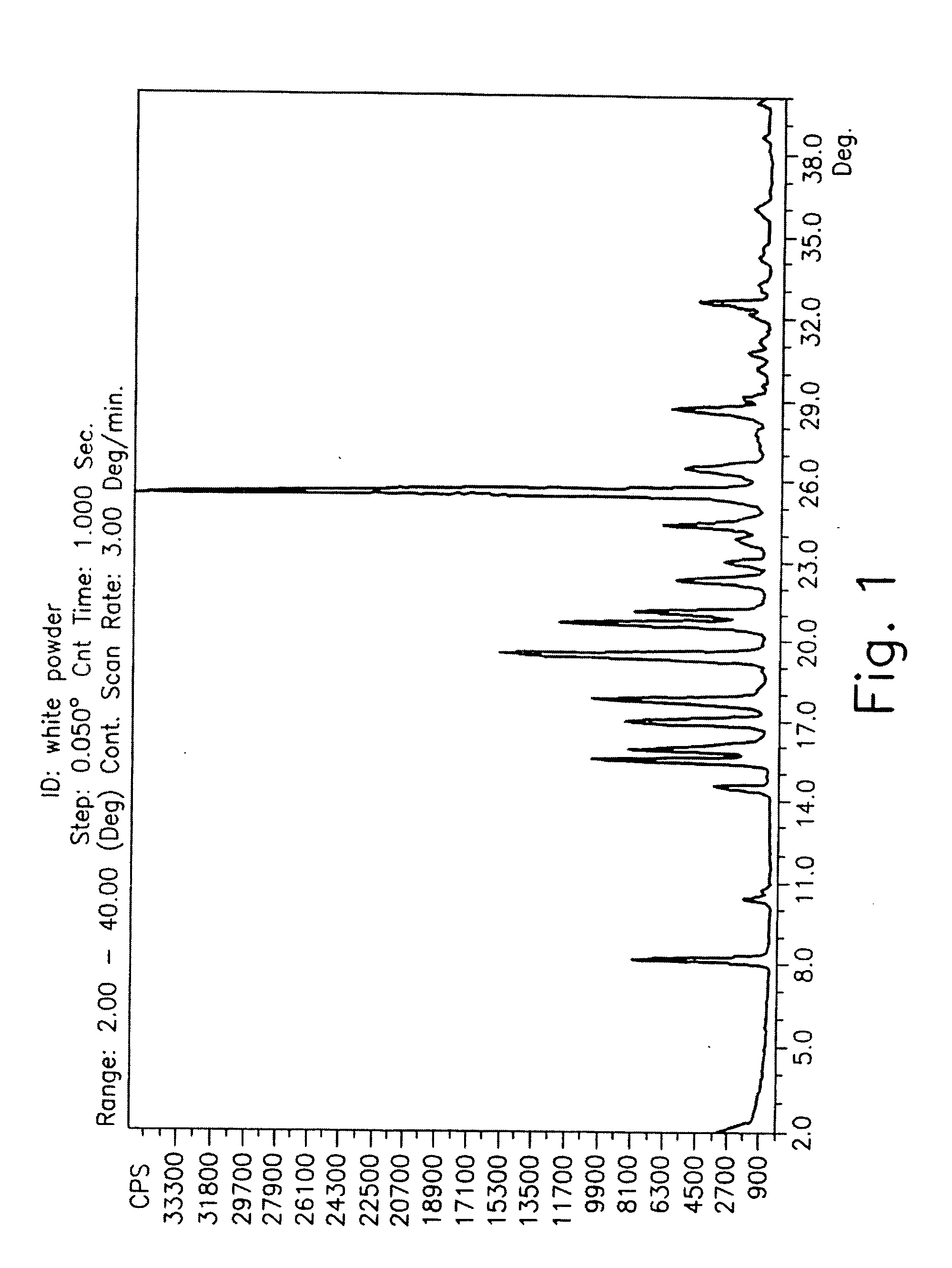
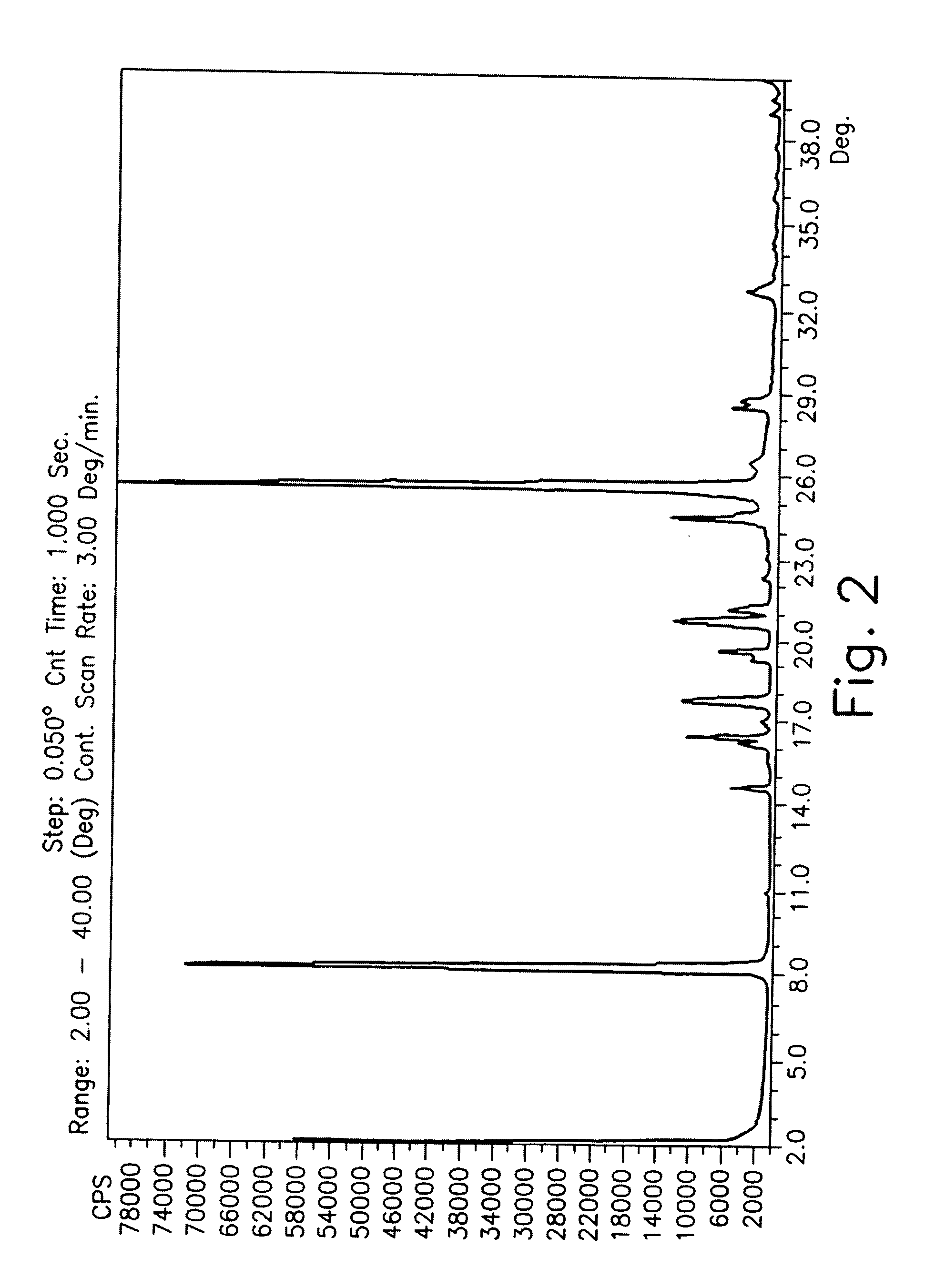
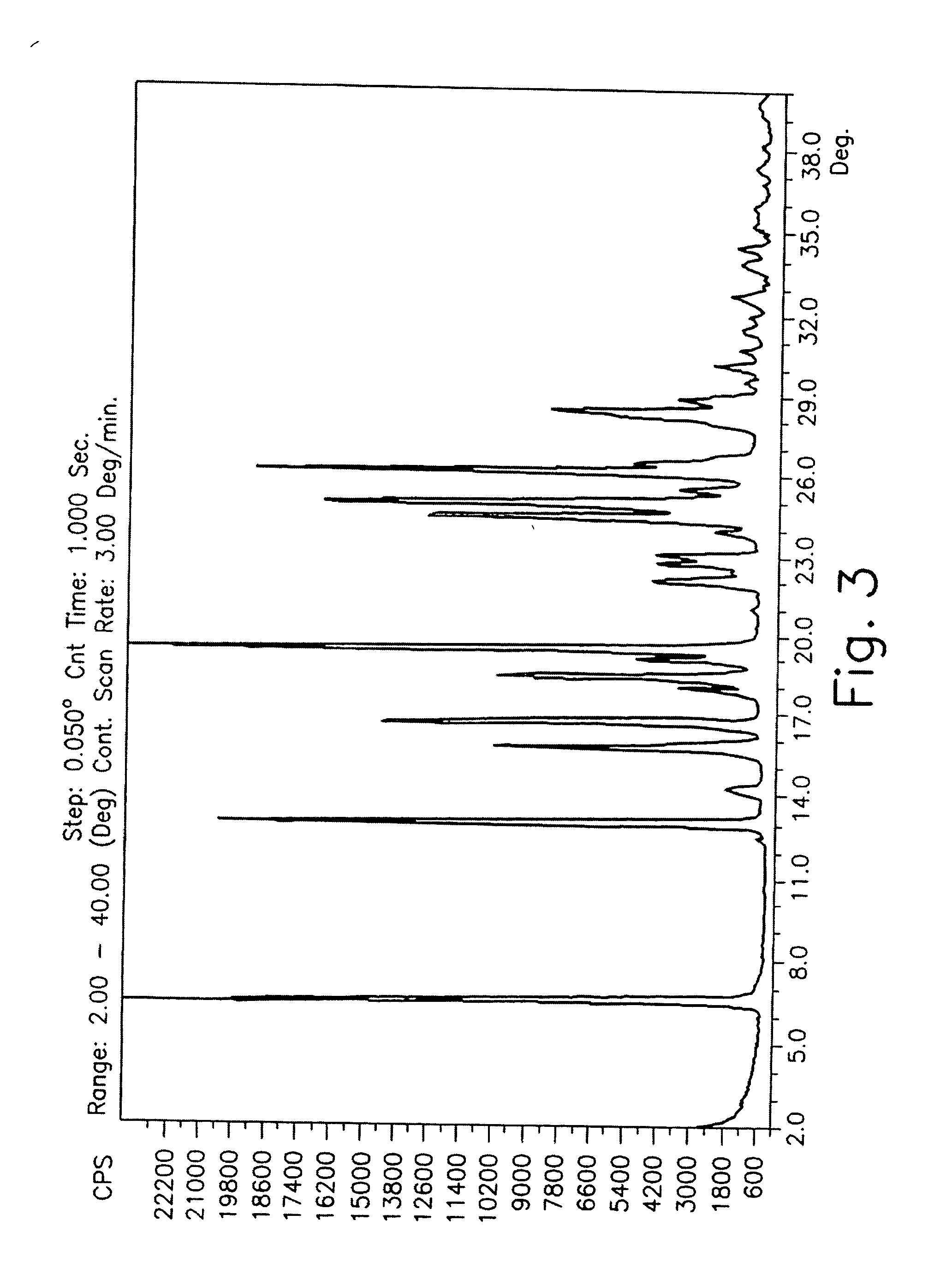
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
65 results about "Famciclovir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Famciclovir is used to treat infections caused by certain types of viruses. It treats shingles caused by herpes zoster.
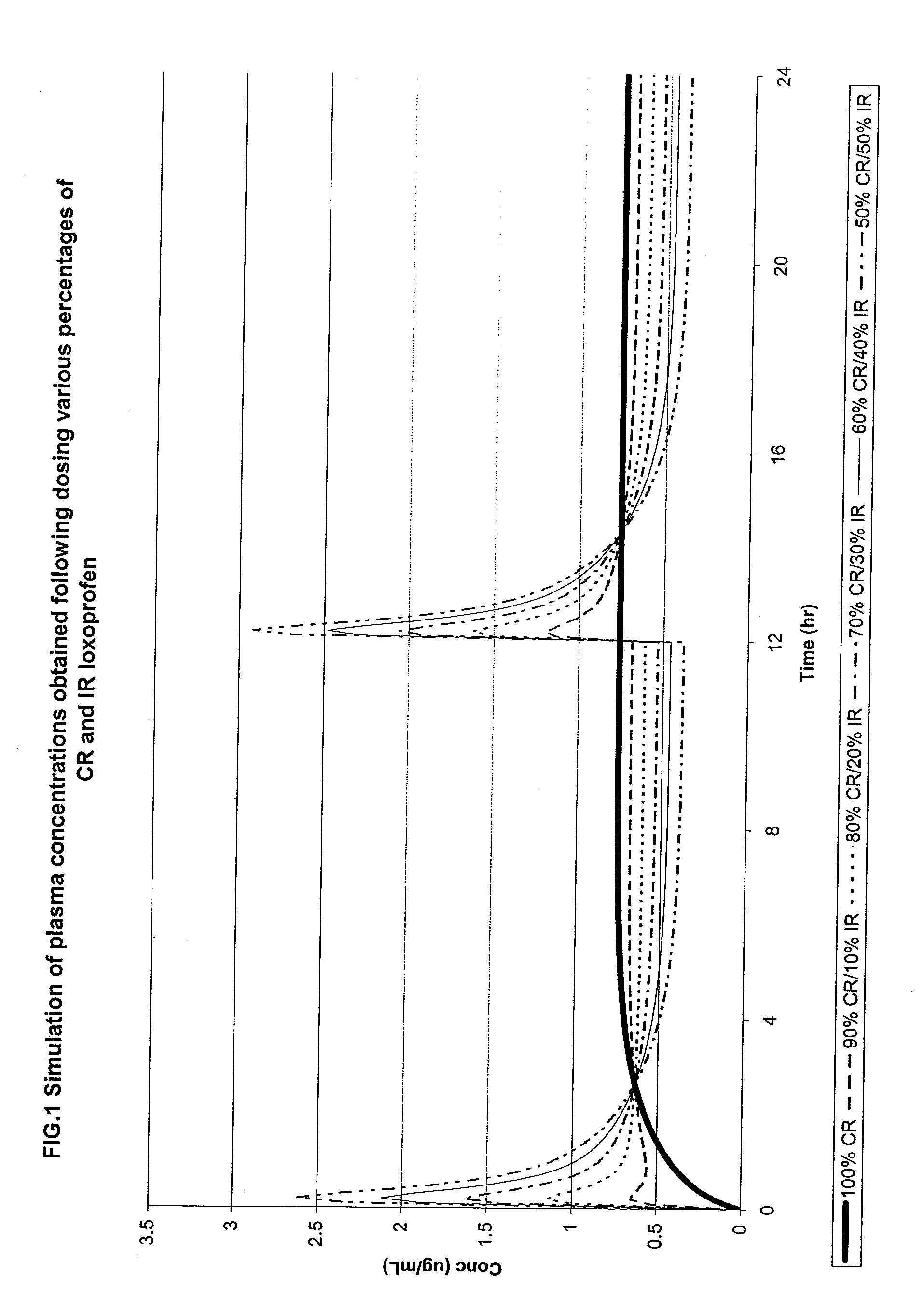
Modified Release Famciclovir Compositions
InactiveUS20100136106A1Reduce dosing frequencyReduce frequencyBiocideAntiviralsBULK ACTIVE INGREDIENTActive ingredient
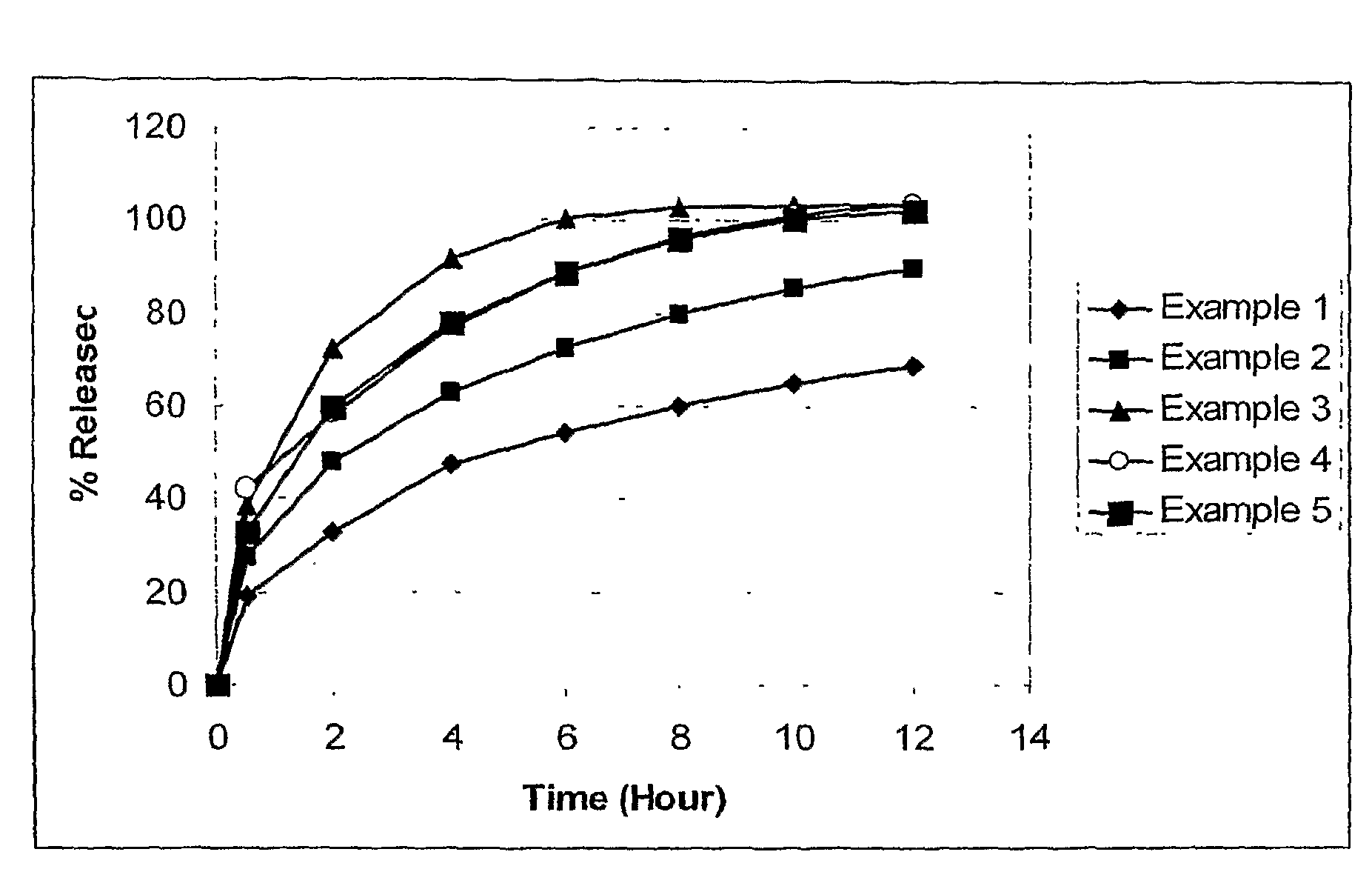
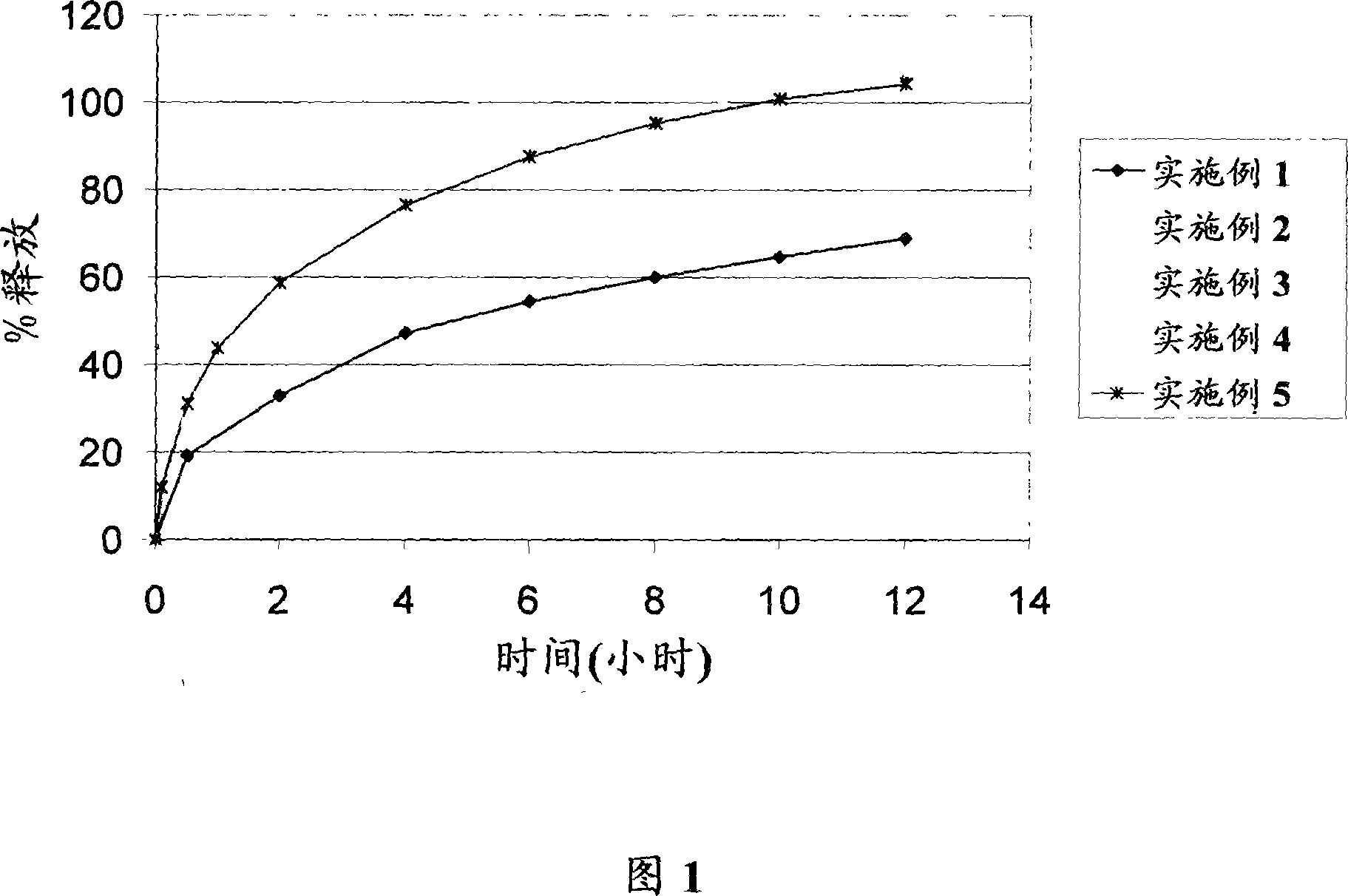
The invention relates to a multiparticulate modified release composition that, upon administration to a patient, delivers famciclovir in a bimodal, multimodal or continuous manner. The multiparticulate modified release composition comprises a first component and at least one subsequent component, the first component comprising a first population of active ingredient containing particles and the at least one subsequent component comprising a second population of active ingredient containing particles. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition, and to a method for the treatment or suppression of viral infections.
Owner:ELAN PHRMA INT LTD
Famciclovir dispersible tablet and preparation method thereof
ActiveCN101904825AAntiviralsPharmaceutical non-active ingredientsCurative effectCombinatorial chemistry
The invention provides a famciclovir dispersible tablet containing famciclovir, disintegrating agents, adhesives and a proper amount of lubricants. Compared with the common famciclovir dispersible tablet, the famciclovir dispersible tablet prepared in the method by adopting a secondary granulation method has the advantages of rapid effect taking and definite curative effect.
Owner:JIANGSU CHENPAI PHARM GRP CO LTD
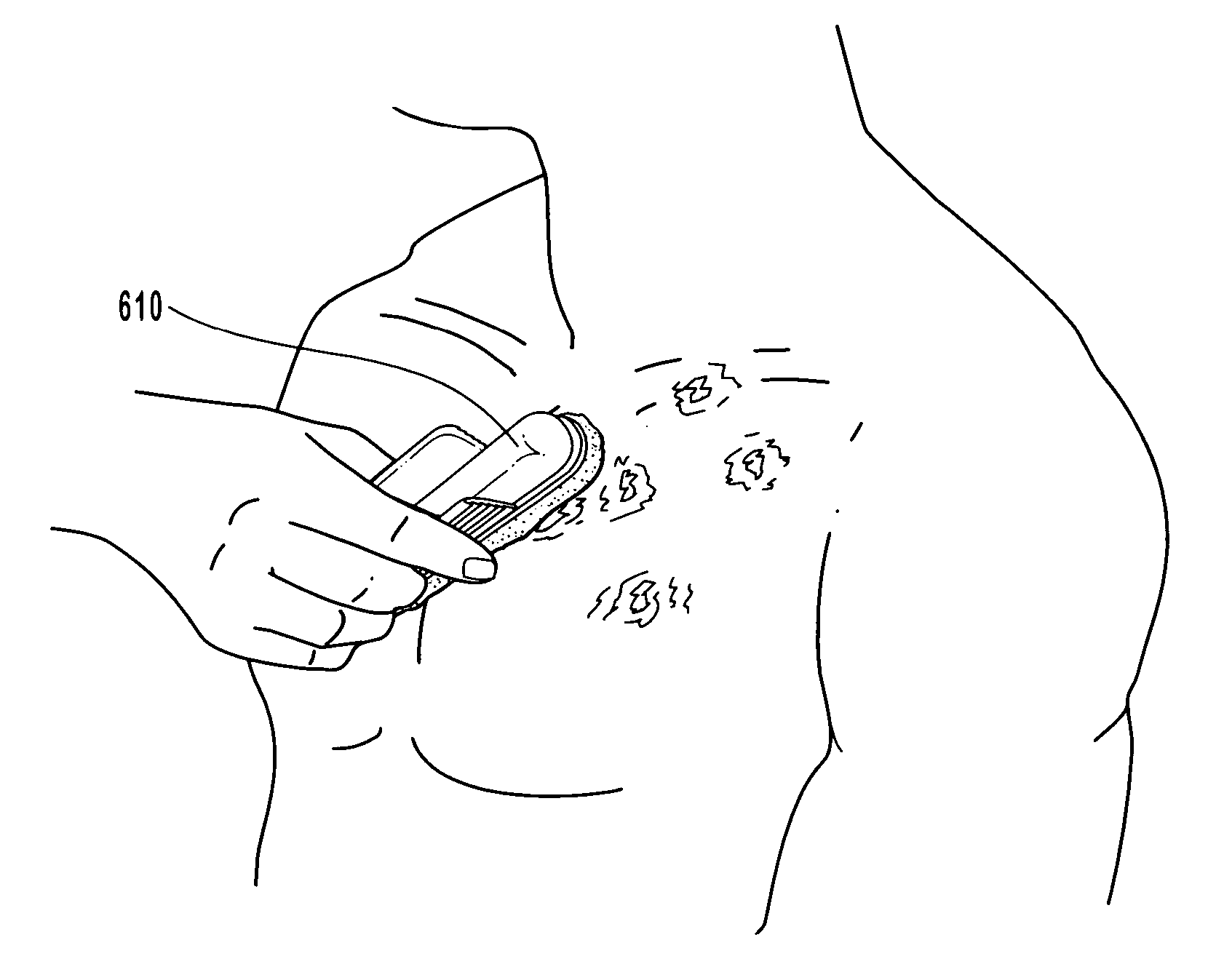
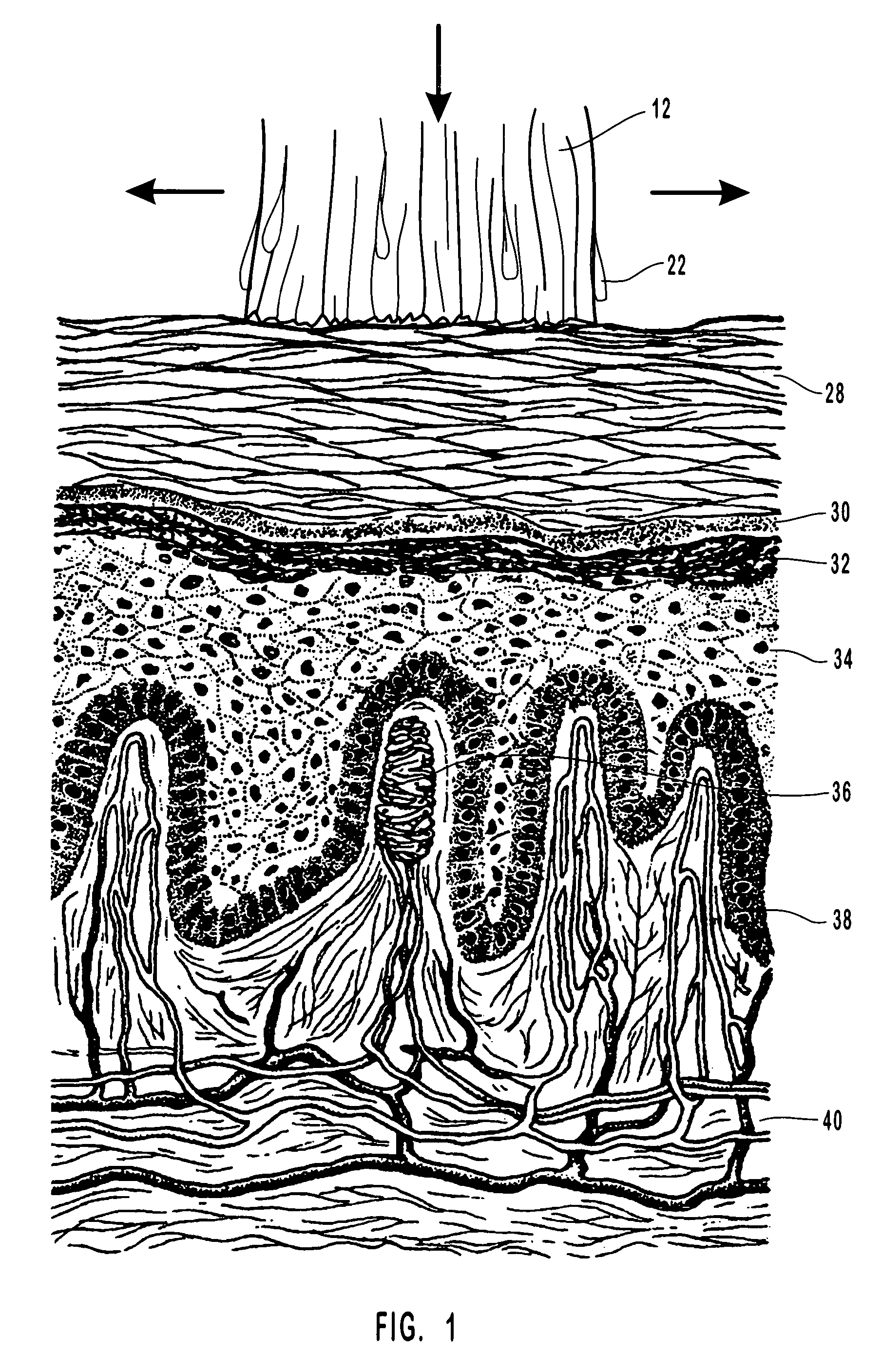
Anti-infective compositions, methods and systems for treating pathogen-induced disordered tissues
InactiveUS20060135464A1Stimulates rapid immunological attackExtraordinary therapeutic effectBiocidePharmaceutical delivery mechanismCidofovirMetabolite
Compositions, methods and systems for treating disordered epithelial tissues, such as is caused by pathogens and / or by toxins produced thereby. The invention relates to the use of an anti-infective and / or antimicrobial active agent in a carrier, with vigorous agitation of the disordered epithelial tissue for topical treatment thereof under such conditions sufficient to achieve clinically discernable improvement of the disordered epithelial tissue. The preferred anti-infective and / or antimicrobial active agent comprises a nucleoside, such as acyclovir, valcyclovir, penciclovir, famciclovir, ganciclovir, cidofovir, adefovir, and tenofovir, and derivatives, analogs, or metabolites thereof, or a mixture thereof, or 1-docosanol, optionally in combination with an organohalide. The inventive compositions and methods may employ the use of an applicator adapted for use in promoting the penetration of the treatment composition and / or the vigorous agitation of the disordered tissue.
Owner:QUADEX PHARMA
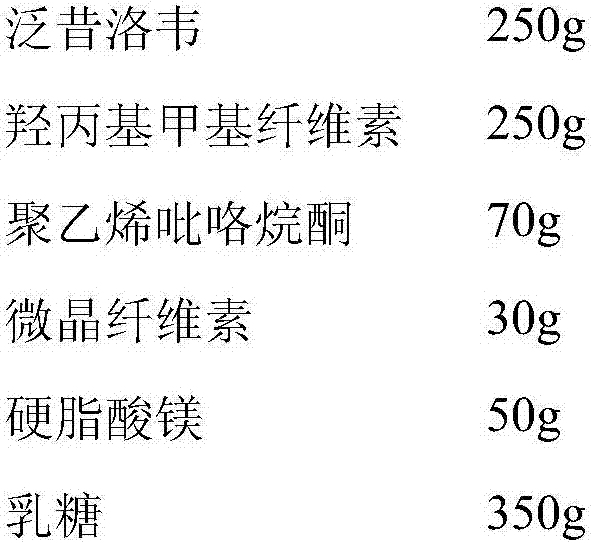
Famciclovir sustained-release pellet, preparation method and application thereof
ActiveCN102920663AFast release rateLess irritatingDigestive systemAntiviralsSustained release pelletsDrug release rate
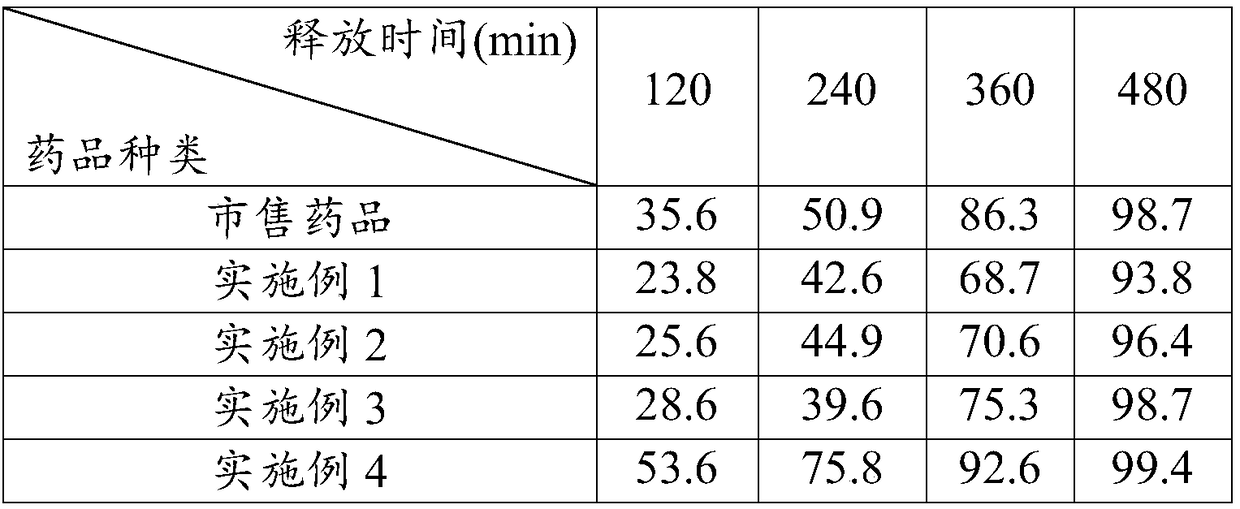
The invention provides a famciclovir sustained-release pellet, a preparation method and an application thereof. The famciclovir sustained-release pellet provided by the invention is composed of a blank pellet core used as a core, a main drug layer containing famciclovir and coating the pellet core, and a sustained-release coating layer coating the main drug layer; the weight of polymer contained in the sustained-release coating layer is 8-15% of the total weight of the blank pellet core and the main drug layer. The famciclovir sustained-release pellet is prepared by a spraying method. According to the famciclovir sustained-release pellet provided by the invention, not only the high drug release rate of the famciclovir sustained-release pellet in an acidic medium is significantly inhibited, but also the final complete release is not influenced; and the following release degree scopes can be realized: 10-30% (1.5 hours), 30-70% (3 hours), and not less than 90% (8 hours).
Owner:LIVZON PHARM GRP INC
Falacyclovir dispersion piece and its preparation method
InactiveCN101156856AShort disintegration timeGood dispersionDigestive systemAntiviralsCross-linked polyethyleneSilica gel
The invention discloses a famciclovir dispersible tablet which is characterized in that the famciclovir dispersible tablet consists of components of the following weight percentages: 40 to 55 percent of famciclovir, 15 to 35 percent of pregelatinized starch, one or more than one of microcrystalline cellulose and lactose, 15 to 30 percent of sodium carboxymethyl starch, one or more than one of low-substituted hydroxypropyl cellulose, crosslinked polyvinyl pyrrolidone, and crosslinked sodium carboxymethyl cellulose, 0.3 to 1 percent of saccharin sodium or aspartame, 0.25 to 1 percent of magnesium stearate, 0.25 to 1 percent of talcum powder or micro-powder silica gel, and appropriate amount of polyvinyl pyrrolidone. The dispersible tablet of the invention has unique performances of short disintegration time, good dispersed state, rapid drug dissolution, high bioavailability and convenience for administration, the production process does not need special equipment, and the production cost is lower.
Owner:刘全胜
Famciclovir slow-releasing capsule for anti-virus treatment and its producing method
ActiveCN1714793ASlow blood concentrationBlood levels rise slowlyDigestive systemPharmaceutical delivery mechanismAnti virusDrug content
The present invention relates to a kind of slow-releasing famciclovir capsule for antiviral treatment, and features that the slow-releasing famciclovir capsule is prepared through coating famciclovir medicine with supplementary material to form the pellet core, coating the pellet core with inner release controlling layer of water insoluble polymer, re-coating with outer release controlling layer of enteric polymer to form release controlling famciclovir pellet, and encapsulating famciclovir pellet to form the slow-releasing famciclovir capsule. In artificial gastric juice and artificial intestinal juice, the slow-releasing famciclovir capsule has its medicine releasing degree of 10-30 %, 30-70 % and over 70 % separately in 1.5 hr, 3 hr and 8 hr. The pellet is measured to have medicine content of 56.8-65.2 %.
Owner:LIVZON PHARM GRP INC
Method for synthesizing famciclovir
InactiveCN101550137AFew reaction stepsRaw materials are cheap and easy to getOrganic chemistryAntiviralsEthyl groupAcetic oxide
The invention relates to a method for synthesizing famciclovir, which includes preparing sodium salt of diethyl malonate at the acting of sodium ethylate by taking diethyl malonate as raw charge and ethyl hydrate as dissolvent, preparing bromo ethyl group diethyl malonate by carrying out nucleophilic substitution reaction with 1, 2-dibromoethane and sodium salt of diethyl malonate, preparing bromo ethyl group propanediol by deoxidizing bromo ethyl group diethyl malonate at the acting of sodium borohydride, preparing 2-acetyl oxygen radicel methyl radicel -4-bromo butyl acetic ester by carrying out reaction with bromo ethyl group propanediol and acylating agent acetic oxide, preparing 2-(2-acetyl oxygen radicel-4-bromo butyl acetic ester)-6-chloropurine by carrying out condensation with 2-acetyl oxygen radicel methyl radical-4-bromo butyl acetic ester and 2-amidocyanogen-6-chloropurine, and then preparing famciclovir by carrying out dechlorination with 2-(2-acetyl oxygen radicel methyl radical -4-bromo butyl acetic ester)-6-chloropurine. This method is suitable for the industrial production with short production line, high yield and low cost.
Owner:彭洋
Famciclovir tablet and preparation method thereof
InactiveCN103462926AInorganic active ingredientsPharmaceutical delivery mechanismSodium bicarbonateMedicine
The invention discloses an antivirus famciclovir tablet. According to the tablet, an aqueous solution of famciclovir and sodium bicarbonate serves as a wetting agent. The tablet is prepared through the wet granulating and tabletting of the following ingredients in parts by weight: 100 parts of famciclovir, 40-65 parts of sodium bicarbonate, 80-200 parts of bulking agent and an appropriate amount of lubricant. Compared with the prior art, the tablet and the preparation method thereof have the advantages that the tablet can be rapidly disintegrated and dissolved without adding a disintegrant, and meanwhile, the preparation method is simple and is suitable for the requirements for large-scale production.
Owner:ZIBO DINGLI PATENT INFORMATION CONSULTING CO LTD
Preparation of famciclovir and other purine derivatives
InactiveUS20060264629A1High yieldHigh regional selectivityOrganic chemistryAntiviralsSide chainPurine
Purine derivatives, substituted at the 9-position, are prepared from a chloro substituted purine starting material, first making an alkyl substitution at the 9-position, then forming the desired esterified side chain, reducing this and hydrogenating the resultant diol prior to addition of alkyl carbonyl groups.
Owner:ARROW INT INC
Process for preparing purine derivative
InactiveUS20100137592A1High selectivityReduce in quantityOrganic compound preparationAmino compound preparationPurinePurine derivative
Owner:AUROBINDO PHARMA LTD
Treatment of hepatitis B infection with thymosin alpha 1 and lamivudine
InactiveCN1320041AEffective treatmentCombined securityPeptide/protein ingredientsPharmaceutical delivery mechanismDrug regimenFamciclovir
A method of treatment of hepatitis B virus (HBV) infection in a patient by administering to the patient a drug regimen including an antiviral-effective amount of thymosin alpha 1 (Talpha1), an antiviral-effective amount of lamivudine, and optionally an antiviral-effective amount of famciclovir is disclosed.
Owner:SCICLONE PHARMACEUTICAL INC
Famciclovir sustained-release dropping pill and preparation method thereof
InactiveCN101269025AImprove accuracyHigh rounding ratePharmaceutical delivery mechanismAntiviralsDrug contentDrug compound
The invention discloses an antivirus drug compound and particularly relates to an oral pharmaceutical preparation adopting famciclovir as the ingredient and being prepared jointly with the medicinal carrier used as the stroma. The antivirus drug compound aims to supplement the deficiency of the prior antivirus oral pharmaceutical preparation and provide a sustained-release famciclovir dropping pill which has high bioavailability, fast effect, high drug content, low frequency of drug taking, full release, convenient drug taking, low cost and absence of contamination during the production. The sustained-release famciclovir dropping pill adopts famciclovir as the ingredient and is prepared jointly with the medicinal carriers of mixing hydrophilic frame ingredients and hydrophobic frame ingredients as the stroma.
Owner:北京博智绿洲医药科技有限公司
Preparation method of famciclovir
ActiveCN109456329AMild reaction conditionsSimple and safe operationOrganic chemistryReaction intermediateSodium nitrite
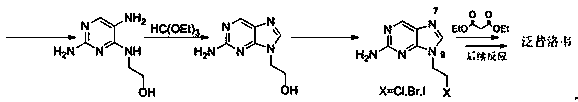
The invention relates to a preparation method of famciclovir. The preparation method comprises the following steps of: adopting guanidine nitrate and diethyl malonate as raw materials, carrying out ring-closing reaction under an alkaline condition to obtain 2-amino-4,6-pyrimidinediol, then obtaining 2-amino-4,6-dichloropyrimidine by hydroxyl chlorination, reacting with 2-(2,2-dimethyl-1,3-dioxan-5-yl)ethyl-1-amine to generate 6-chloro-N(i)4( / i)-(2-(2,2-dimethyl-1,3-dioxan-5-yl)ethyl)pyrimidine-2,4-diamine, then reacting with sodium nitrite under the acidic condition to obtain 2-(2-((2-amino-6-chloro-5-nitrosopyrimidin-4-yl)amino)ethyl)propane-1,3-diol, and finally carrying out reduction / dechloridation, ring-closing and esterification reaction to obtain the famciclovir. The preparation method has the beneficial effects that the problems of poor N-alkylation reaction selectivity and need of additional purification of reaction intermediates and the like in the current process are solved.
Owner:迪嘉药业集团股份有限公司
Process for preparing famciclovir
The invention provides a process for making famciclovir, comprising reacting 9-[4-acetoxy-3-(acetoxymethyl)but-1-yl]-2-amino-6-chloropurine (Cl-FMC) with a palladium on charcoal catalyst in water and ammonium formate. The invention also provides methods of treating viral diseases by administering the famciclovir prepared according to the above process.
Owner:TEVA PHARM USA INC
High-stability famciclovir tablet and preparation method thereof
ActiveCN106511286AHigh dissolution rateLess impuritiesDigestive systemAntiviralsCross-linkSodium bicarbonate
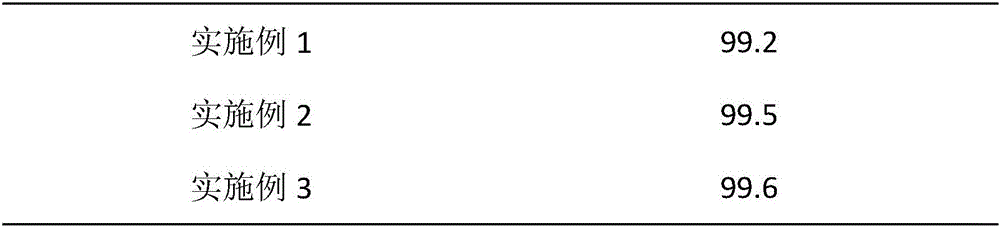
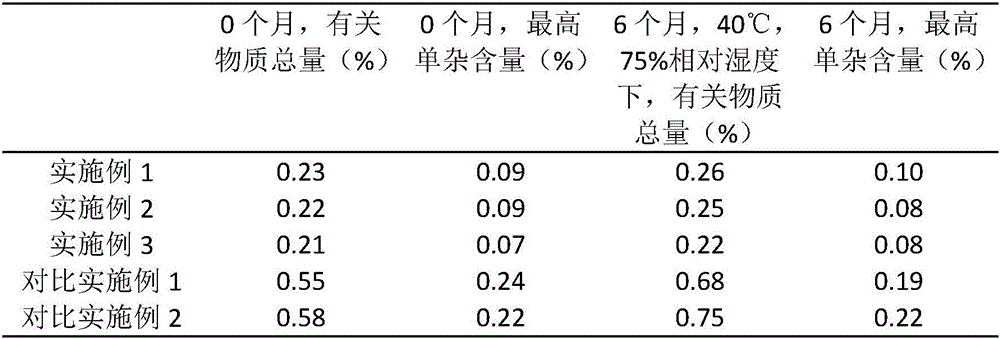
The invention provides a preparation method of a high-stability famciclovir tablet. The method comprises the following steps: (1) mixing famciclovir, mannitol, sodium carboxymethyl starch and sodium bicarbonate according to a weight ratio of (3-5) to (1-1.5) to (0.5-0.6) to (0.8-1); (2) adding a carboxypropyl methyl cellulose ethanol solution, which is 2.8-3.5% in weight percentage, to a material obtained in the step (1), preparing a soft material, preparing granules by virtue of a 14-mesh sieve, drying the granules at 65-75 DEG C, and straightening the granules by virtue of a 20-mesh sieve; and (3) adding magnesium stearate and cross-linked povidone to a material obtained in the step (2), and conducting tabletting, so that the famciclovir tablet is obtained. The invention also provides the famciclovir tablet prepared by the method. The famciclovir tablet provided by the invention is high in dissolution rate, and the dissolution rate, within 5min, can reach 99.2% or above; the famciclovir tablet is low in content of impurities, and a total impurity content is just 0.21-0.23% and an individual impurity content is within 0.10%; and the prepared famciclovir tablet, under an accelerated test condition, is quite high in stability, and the content of impurities is basically not increased.
Owner:四川省百草生物药业有限公司
Application of nucleoside type antiviral medicine to preparation of medicine for treating infarction diseases
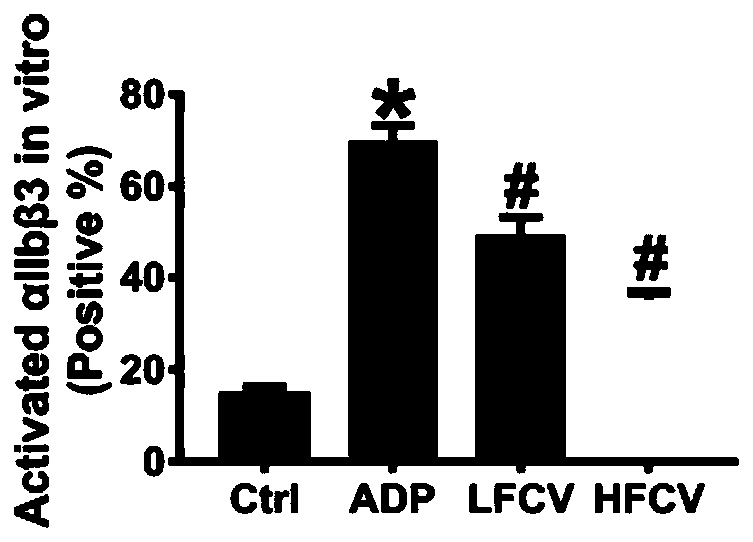
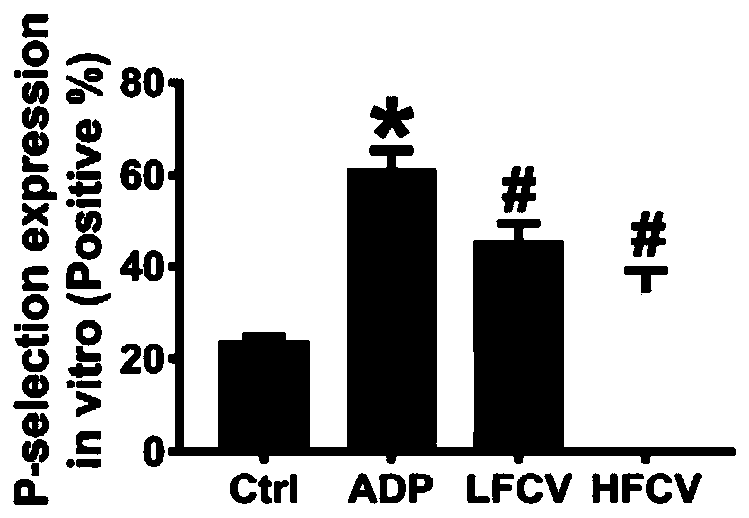
InactiveCN111569075AReduce severityReduce frequencyBlood disorderHeterocyclic compound active ingredientsDiseaseHerpesvirus infection
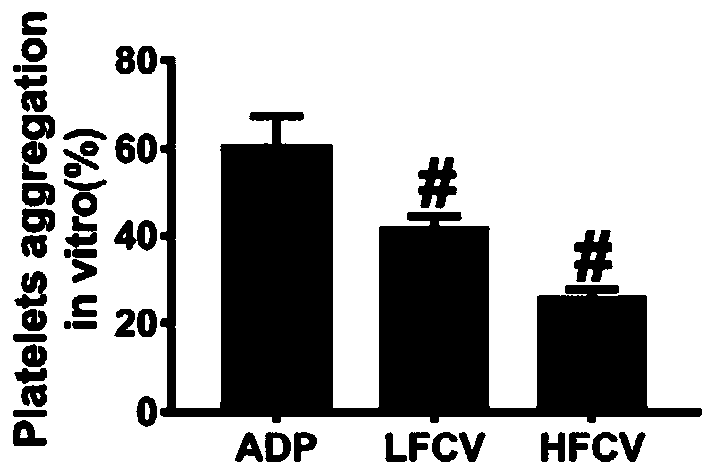
The invention discloses application of nucleoside type antiviral medicine to preparation of medicine for treating infarction diseases, relates to the technical field of medicine, and aims at providinga novel direction for researching and developing the medicine for treating the infarction diseases. According to the application of the nucleoside type antiviral medicine to preparation of the medicine for treating the infarction diseases provided by the invention, the nucleoside type antiviral medicine can reduce the serious degree and the frequency of herpes simplex virus infection, and also has the significant activity of relieving infarction complications. Studies prove that in the infarction diseases, blood platelet adhesion, activation and aggregation achieve the key regulating effects;the nucleoside type antiviral medicine, such as famciclovir can inhibit the blood platelet aggregation, inhibit the GPIIb / IIIa activation, inhibit P-selectin expression and / or inhibit arteriovenous thrombus and cerebral infarction formation; meanwhile, a blood coagulation system is not influenced; and a novel idea is provided for the research and development of medicine for treating the infarction diseases.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
Famciclovir capsule preparation and preparation method thereof
ActiveCN103301089AReduce typesReduce dosageAntiviralsPharmaceutical non-active ingredientsSustained release pelletsWax
The invention discloses a famciclovir capsule preparation which comprises a capsule shell and a content, wherein the content consists of famciclovir sustained-release pellets; the famciclovir sustained-release pellets are prepared through the following steps of: dissolving famciclovir, ethyecellulose and high-substituted hydroxy propyl cellulose in ethanol, adding the components into liquid wax with a surfactant under the stirring condition, gradually raising the temperature to be 38-40 DEG C, reserving the temperature and stirring for 4-8 hours, raising the temperature to be 68-72 DEG C, stirring for 0.5-2 hours, reducing the pressure and filtering so as to obtain the famciclovir sustained-release pellets. By utilizing the preparation disclosed by the invention, not only can the amount of sustained-release material be greatly reduced, but also the phenomenon of burst release of the medicine is eliminated.
Owner:广东彼迪药业有限公司
Method for synthesizing famciclovir
ActiveCN101555249AMild experimental conditionsImprove responseOrganic chemistryAntiviralsCoupling reactionFamciclovir
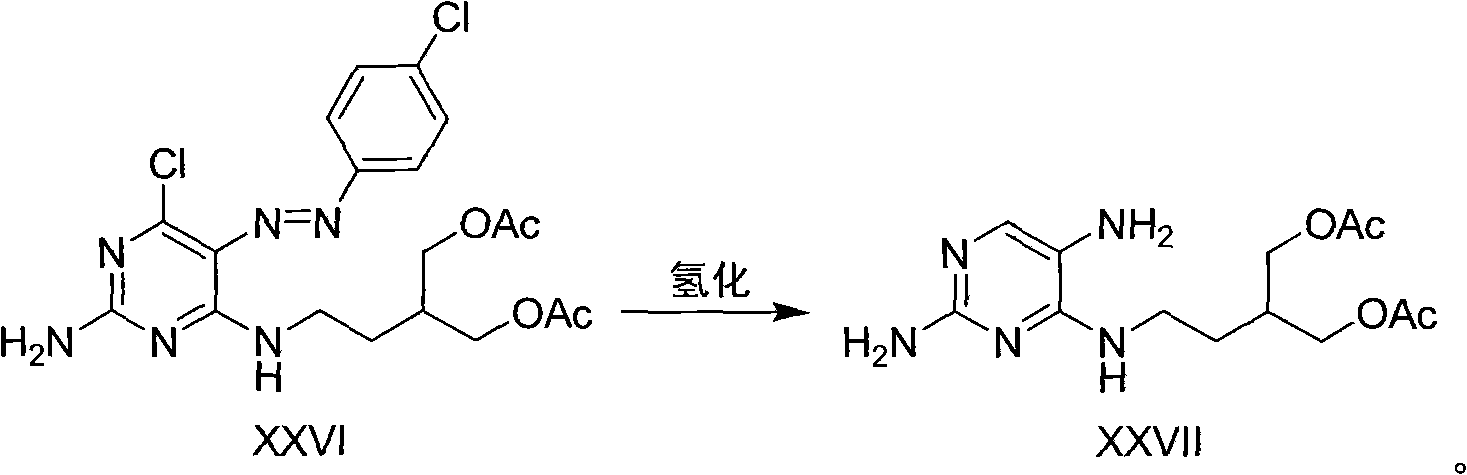
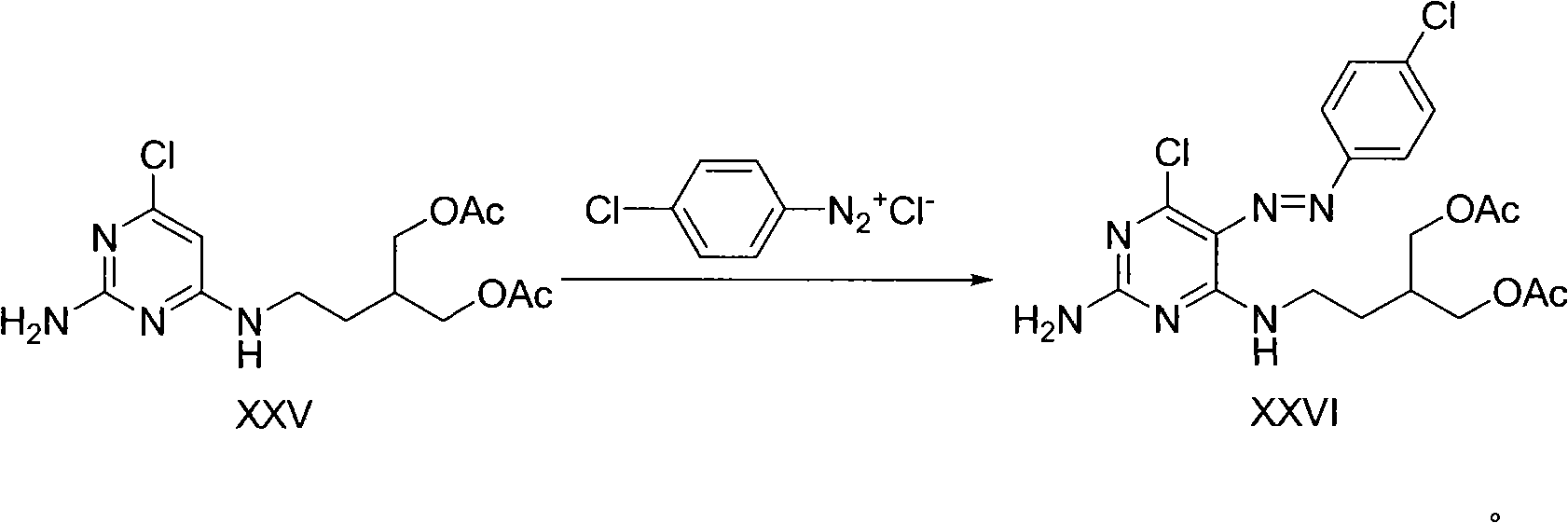
The invention provides a method for efficiently, simply and conveniently synthesizing famciclovir. 2-amidogen-4, 6-dichloro pyrimidine is used as a raw material, and the famciclovir is high selectively synthesized by coupling reaction, acidylation reaction, diazotization reaction, reduction reaction and ring closure reaction of the raw material. The synthesizing raw material is easy to obtain, experiment conditions are moderate, the reactions are easy to control and simple, and a midbody is not purified if permitted not to be purified so that middle operation links are increased, thereby the method is suitable for the industrialized production.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Synthetic method of famciclovir intermediate
A synthetic method of a famciclovir intermediate comprises the following steps: carrying out a condensation of an initial raw material 2,4-dichloro-5-nitropyrimidine and 2-(2-aminoethyl)propylene glycol, carrying out selective substitution of the 4th chlorine atom to obtain 2-{2-[(2-chloro-5-nitropyrimidyl-4-yl)amino]ethyl}-1,3-propylene glycol, and ammonolyzing to obtain 2-{2-[(2-amino-5-nitropyrimidyl-4-yl)amino]ethyl}-1,3-propylene glycol; and further reducing to obtain 2-{2-[(2-amino-5-aminopyrimidyl-4-yl)amino]ethyl}-1,3-propylene glycol, and cyclizing to obtain the famciclovir intermediate 2-[2-(2-amino-9H-purine-9-yl)ethyl]-1,3-propylene glycol. The synthetic method has the advantages of less steps, high yield, effective avoiding of the generation of isomers, simple subsequent purification step, production cost reduction, and suitableness for the industrialized application.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
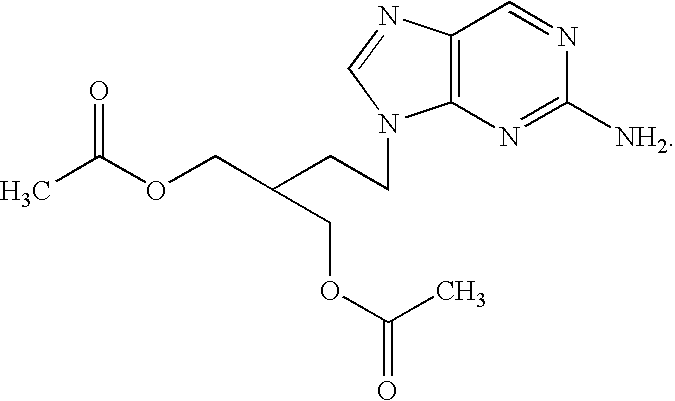
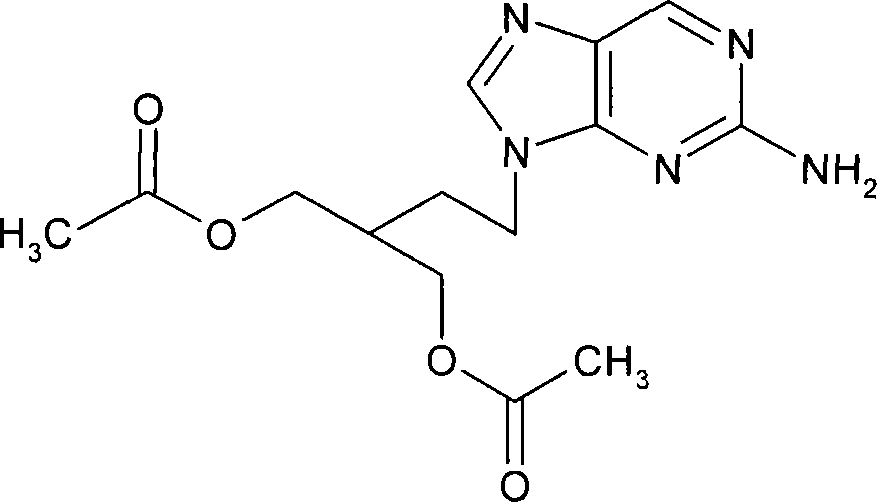
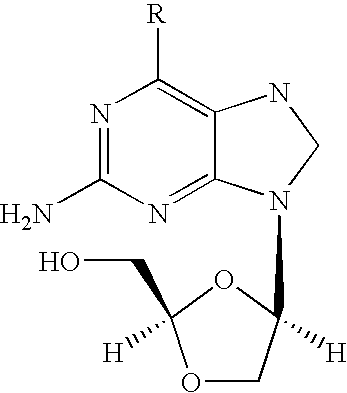
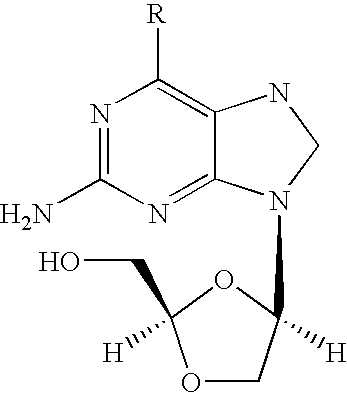
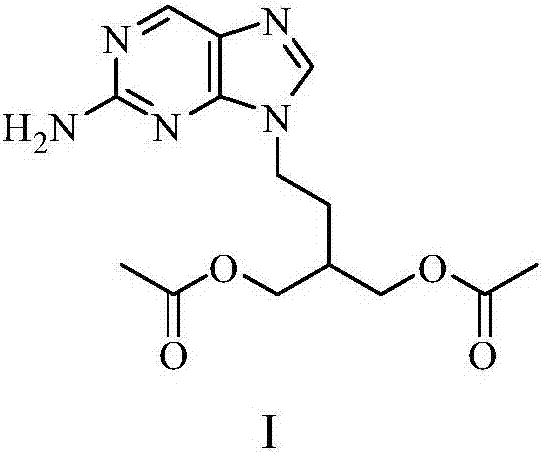
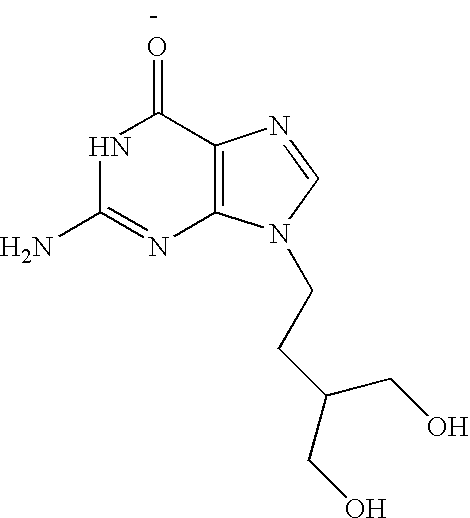
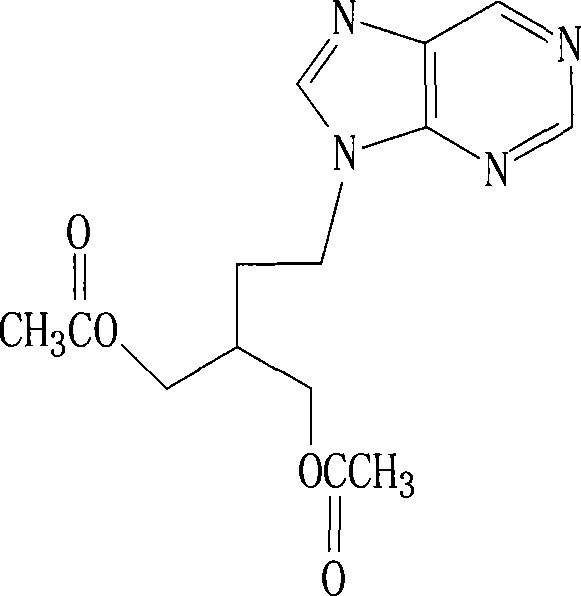
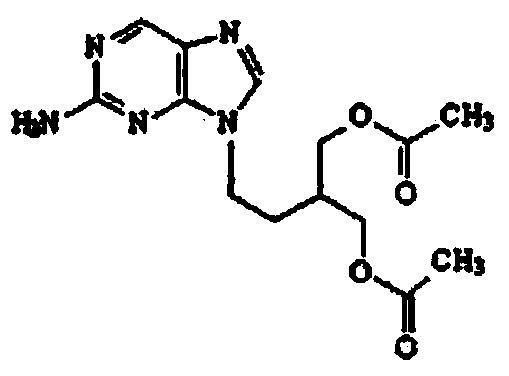
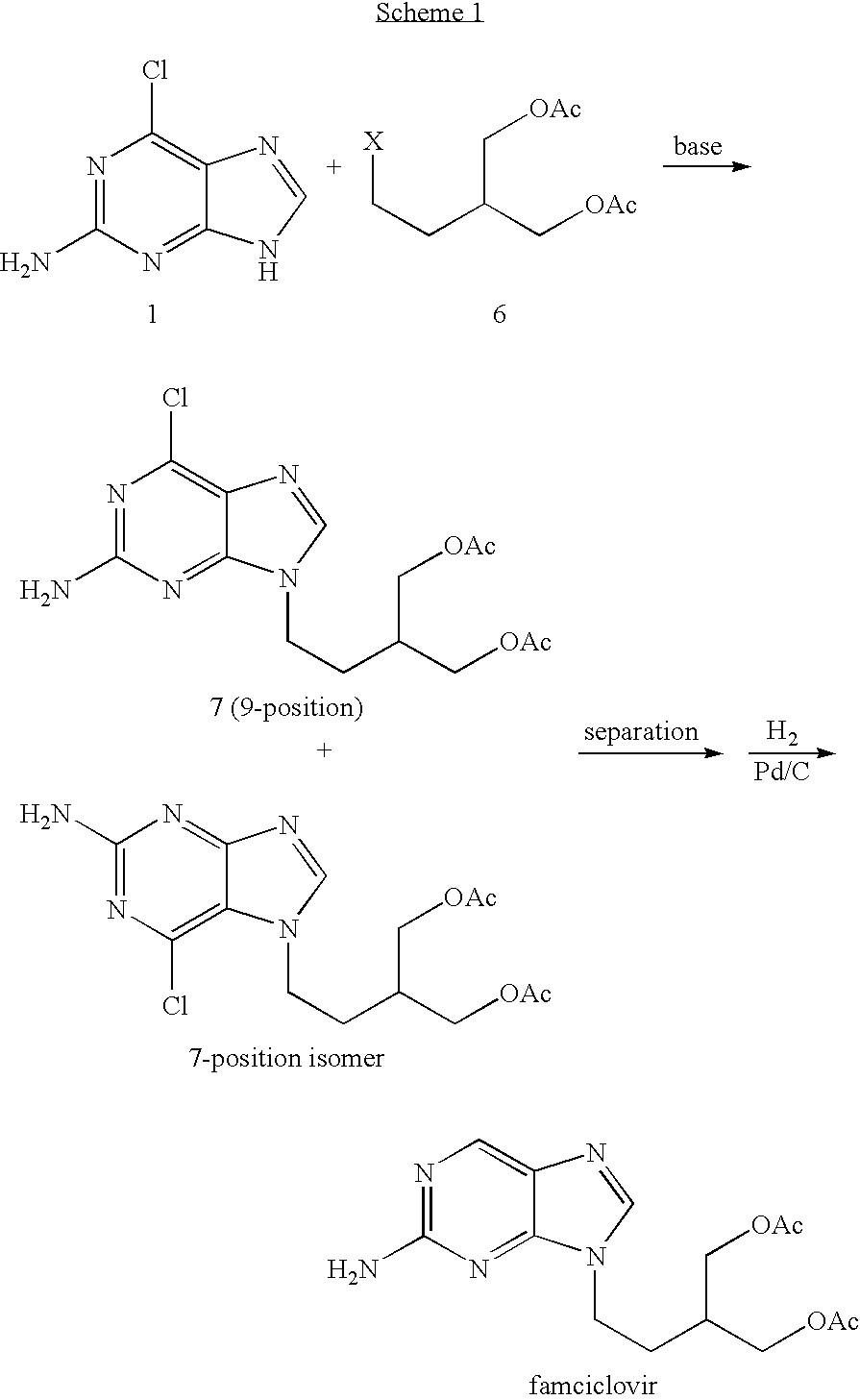
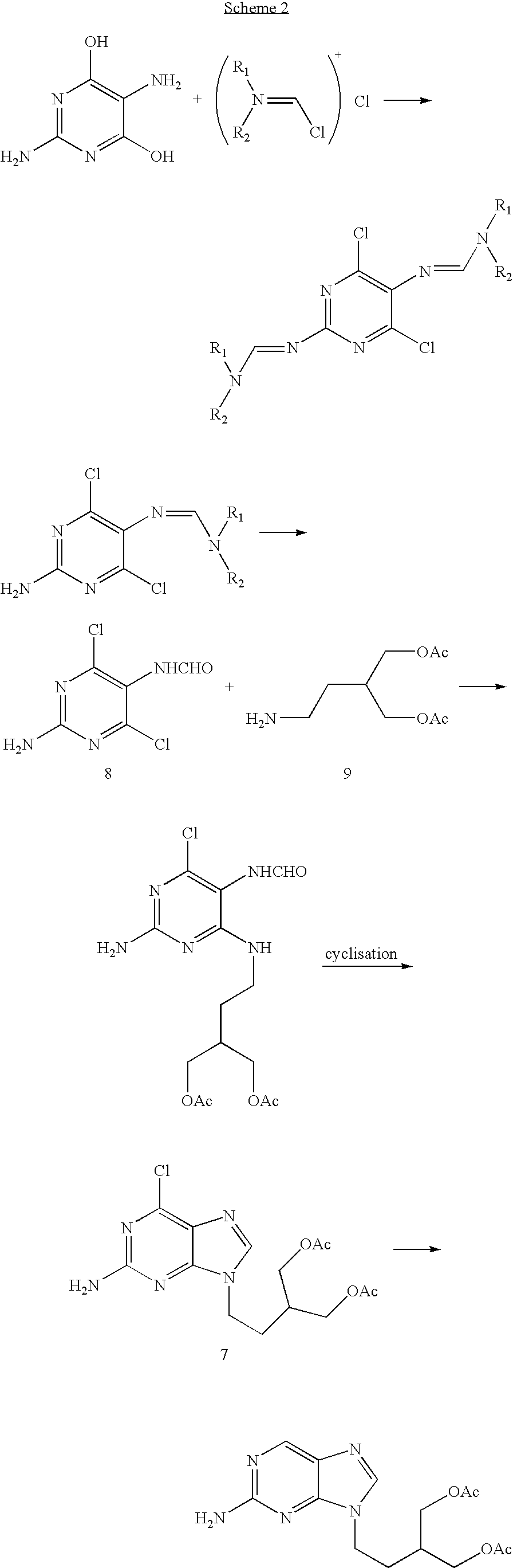
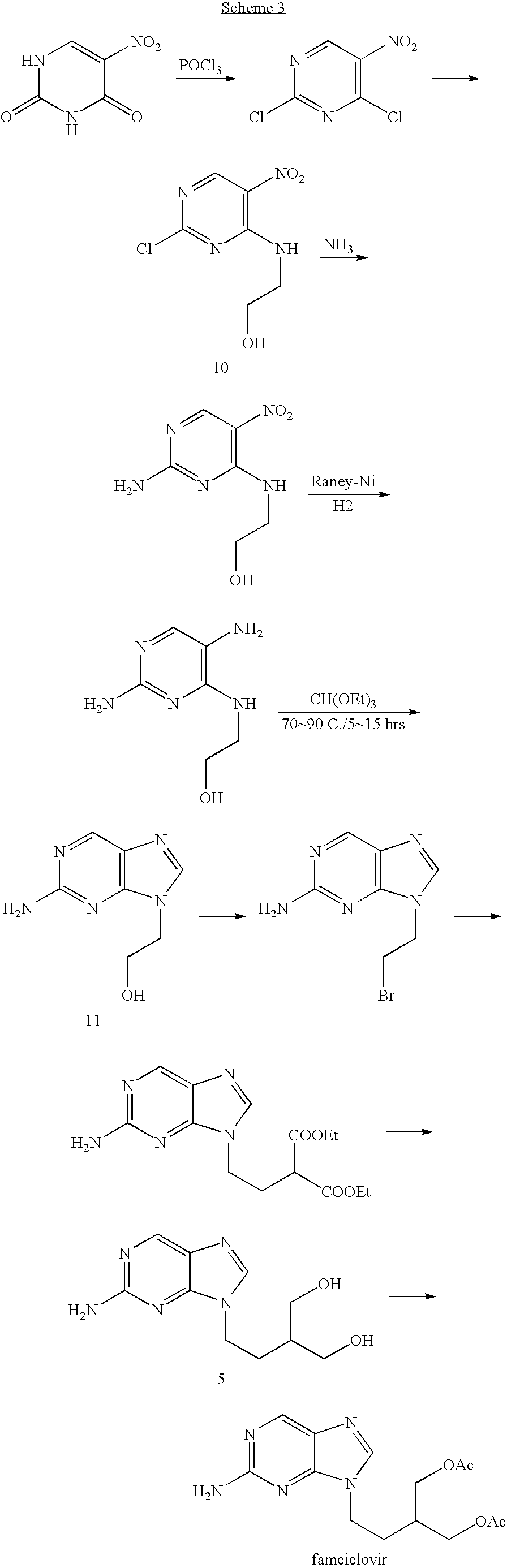
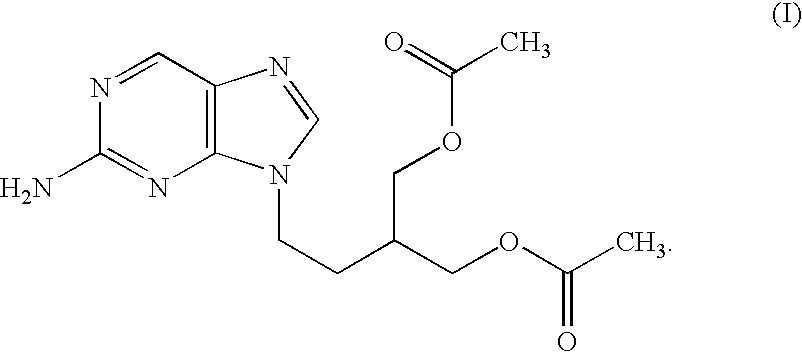
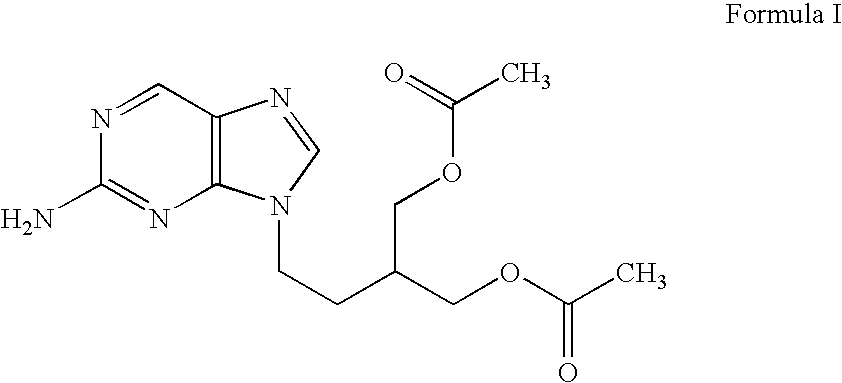
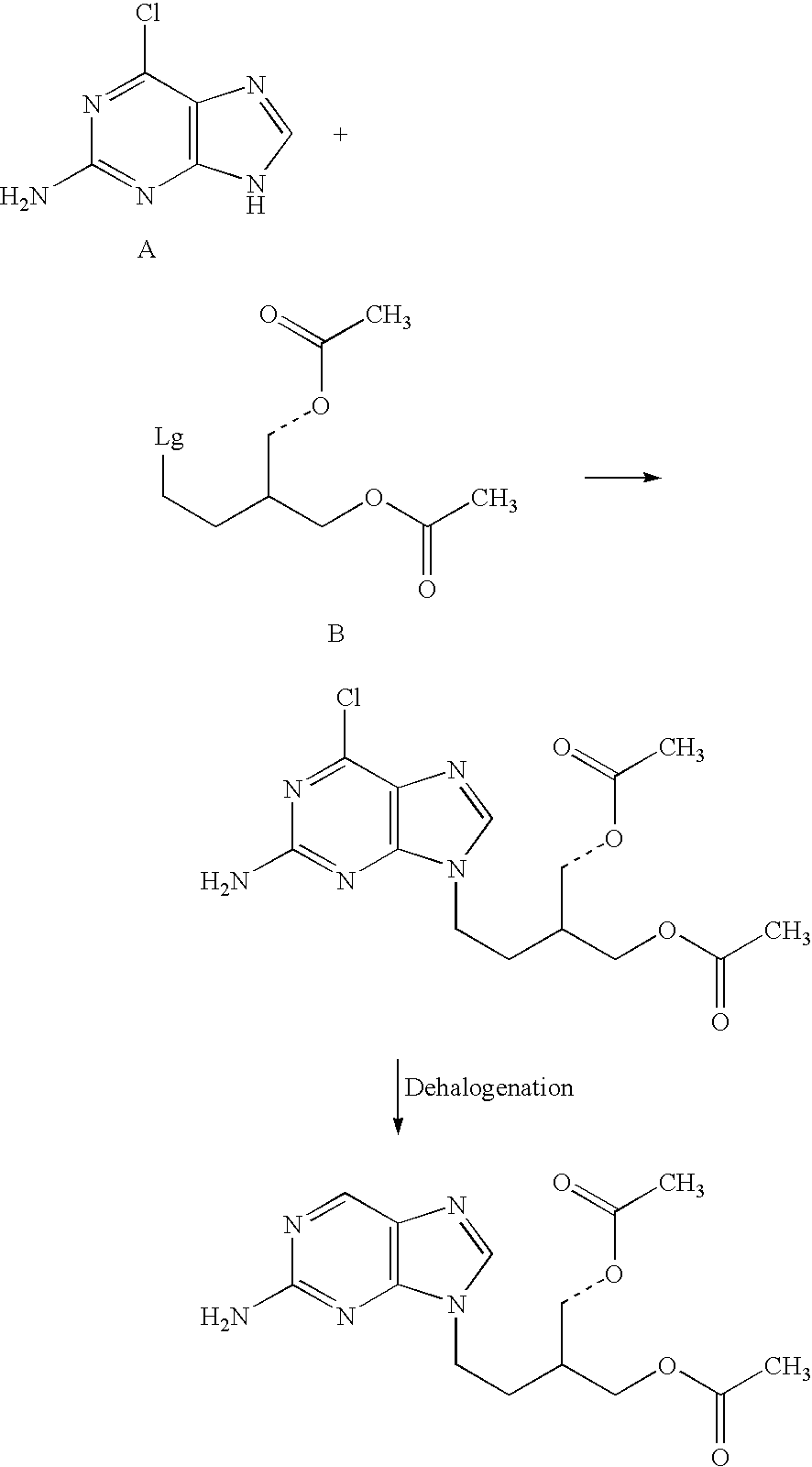
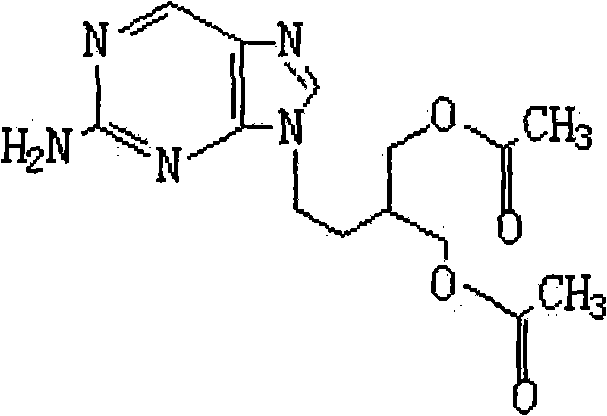
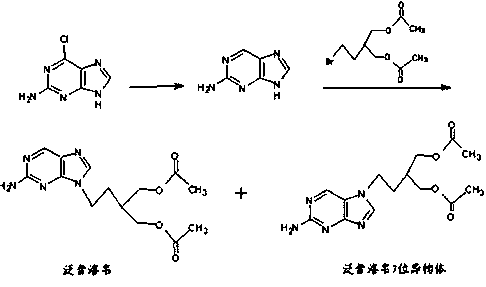
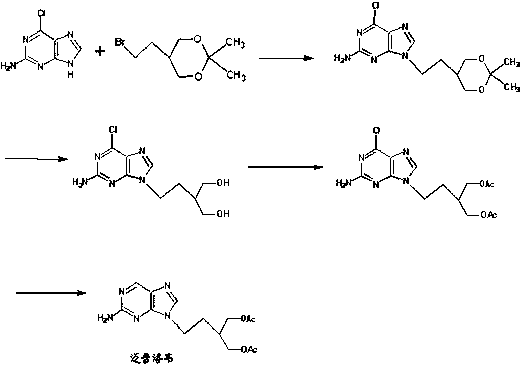
2-amino-9-(2-substituted ethyl)purines and preparing methods for 9-[4-acetoxy-3-(acetoxymethyl)but-1-yl]-2-aminopurine using the same
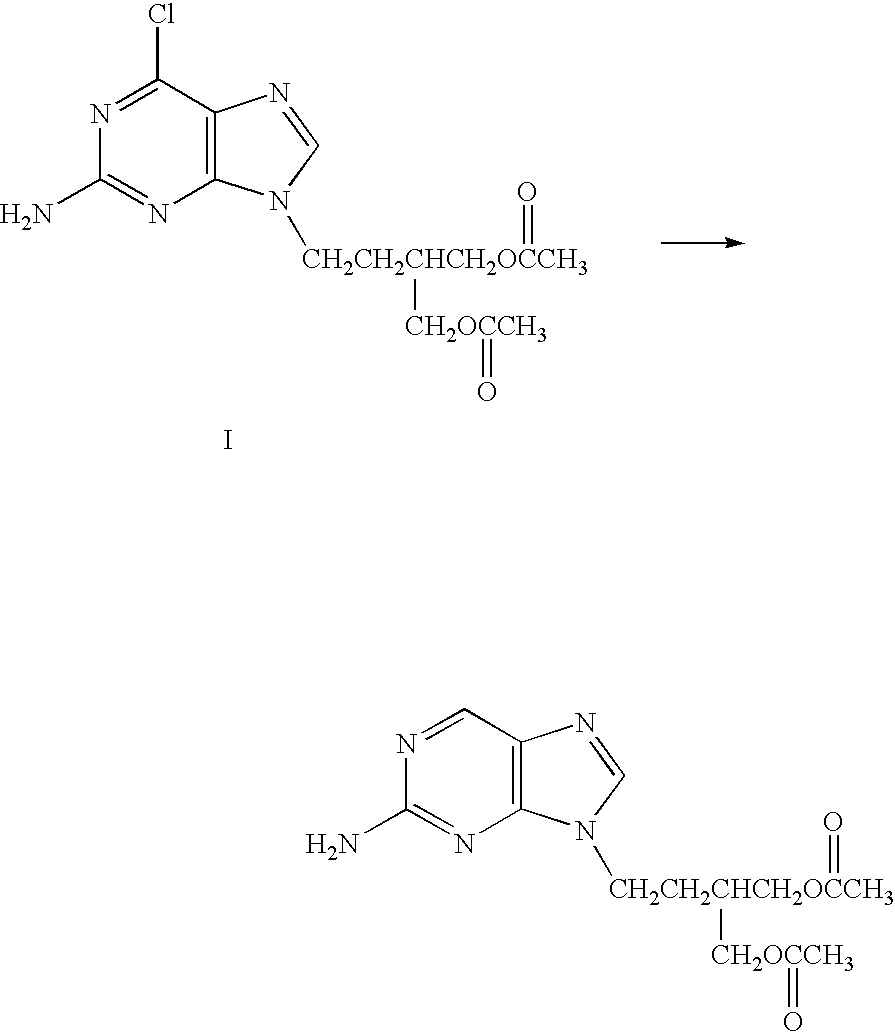
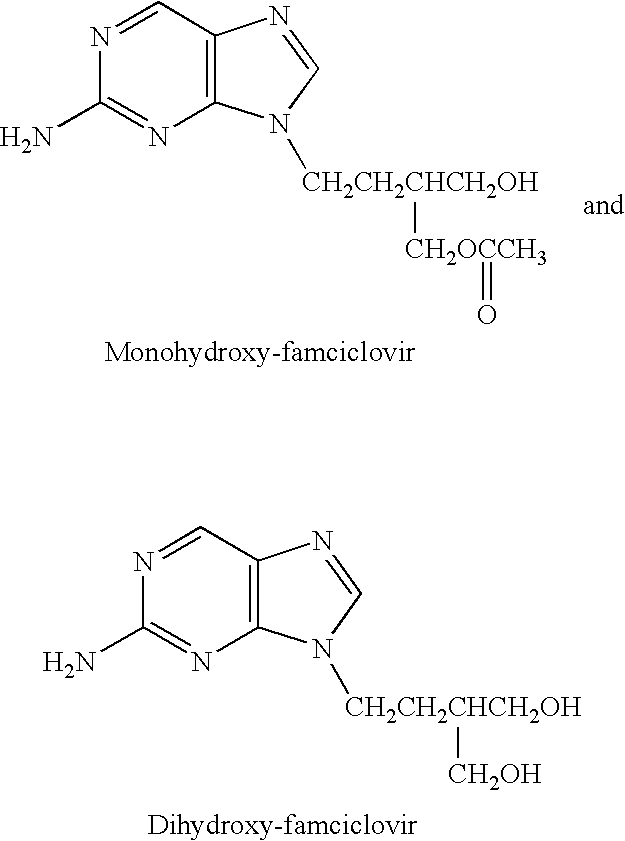
The present invention relates to a new compound of 2-amino-9-(2-substituted ethyl)purine and an effective method for preparing 9-[4-acetoxy-3-(acetoxymethyl)but-1-yl]-2-aminopurin (famciclovir) using the same. The 2-amino-9-(2-substituted ethyl)purine according to the invention is represented by the following formula (II′): (Formula II′) wherein R is a hydroxy, halogen, mesyloxy or tosyloxy group. The inventive method for the preparation of famciclovir comprises the steps of halogenating 2-amino-9-(2-substituted ethyl)purine to give 2-amino-9-(2-halogenoethyl)purine, and reacting the halogenated compound with diethylmalonate. The inventive preparation method allows famciclovir, a purine derivative drug with effective antiviral activity, to be prepared in a high selectivity of 100% in a pure form by using the inventive new compound of 2-amino-9-(2-substituted ethyl)purine. In addition, the inventive method allows the utilization of relatively mild reaction conditions, and thus, has high industrial process efficiency.
Owner:KYUNG DONG PHARM
Modified release famciclovir pharmaceutical compositions
A modified release pharmaceutical composition of famciclovir contains at least 60% by weight famciclovir with at least 5% by weight of a release retardant. Particularly useful as a release retardant include polymers, especially a mixture of polyvinyl acetate and polyvinylpyrrolidone. A method of making such pharmaceutical compositions using a extruder and a granulation method is particularly useful.
Owner:LEE WAI YIP +1
Modified release famciclovir pharmaceutical compositions
A modified release pharmaceutical composition of famciclovir contains at least 60% by weight famciclovir with at least 5% by weight of a release retardant. Particularly useful as a release retardant include polymers, especially a mixture of polyvinyl acetate and polyvinylpyrrolidone. A method of making such pharmaceutical compositions using a extruder and a granulation method is particularly useful.
Owner:NOVARTIS AG
Famciclovir tablet composition
ActiveCN112587491AUniform adhesionImprove moisture resistanceAntiviralsPharmaceutical non-active ingredientsCelluloseCarboxymethyl starch
The invention relates to a famciclovir tablet composition, and belongs to the technical field of pharmaceutical preparations. According to the technical scheme, the famciclovir composition contains the following components in parts by mass of 75.8 parts of famciclovir, 6.7-8.4 parts of lactose monohydrate, 2.4 parts of hydroxypropyl cellulose L, 9.7-11.4 parts of D-glucosamine hydrochloride, 3 parts of carboxymethyl starch sodium and 0.7 part of magnesium stearate. The famciclovir tablet composition with stable performance provided by the invention solves the moisture absorption problem of thefamciclovir.
Owner:DISHA PHARMA GRP
Drying process for preparing crystalline solid famciclovir
The present invention provides a drying process for preparing crystalline solid famciclovir form I comprising the steps of: i) preparing a wet crystalline form of famciclovir; ii) drying the wet crystalline form of famciclovir at a temperature of below 50° C. until the crystalline form contains less than 15% (wt / wt) wetness; and iii) drying the wet crystalline form of famciclovir at a temperature of above 50° C. until the wet crystalline form of famciclovir contains less than 0.05% (wt / wt) water to obtain crystalline solid famciclovir form I.
Owner:TEVA PHARM USA INC
Pharmaceutical composition of famciclovir and pharmaceutical application of pharmaceutical composition
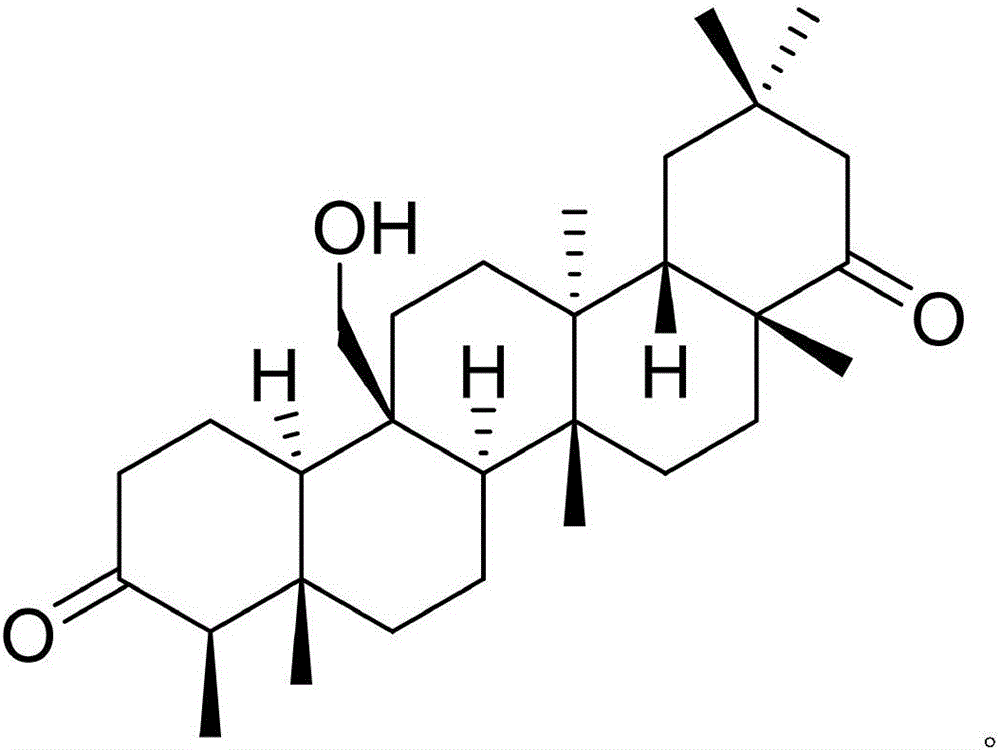
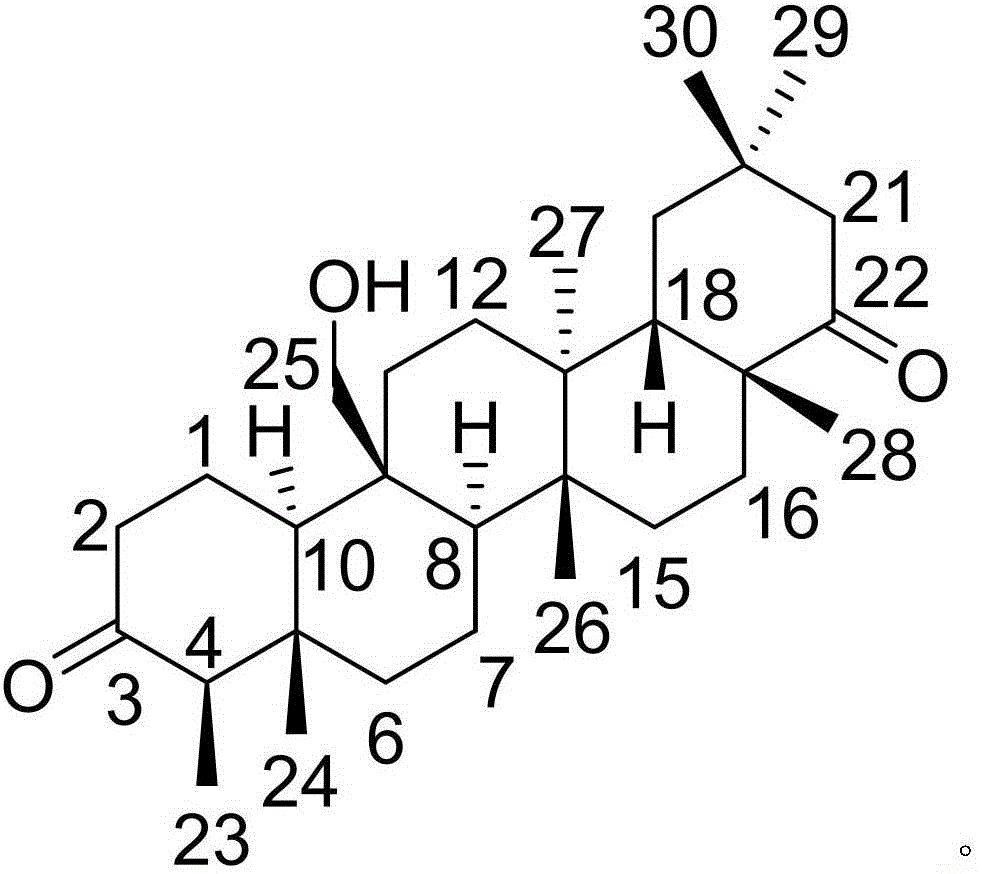
InactiveCN105777850AHas therapeutic effectGood treatment effectNervous disorderSteroidsNatural productTherapeutic effect
The invention discloses a pharmaceutical composition of famciclovir and pharmaceutical application of the pharmaceutical composition. The pharmaceutical composition comprises the famciclovir and a natural product compound (I) of a novel structure; when in single action, the famciclovir and the compound (I) have therapeutic effect in epilepsy; when the famciclovir and the compound (I) are in combined action, the therapeutic effect in epilepsy is further improved; the pharmaceutical composition can be developed into drugs for treating epilepsy; the pharmaceutical composition has outstanding substantive features and significant improvement as compared with the prior art.
Owner:周俭
Combination therapy to treat hepatitis B virus
The present invention is directed to a method for treating hepatitis B virus infection in humans comprising administering a synergistically effective amount of agents having known anti-hepatitis B virus activity in combination or alternation. Specifically, the invention is directed to a method for treating hepatitis B virus infection comprising administering FTC in combination or alternation with penciclovir, famciclovir or Bis-POM-PMEA. Additionally, the invention is directed to a method for treating hepatitis B virus infection comprising administering L-FMAU in combination or alternation with DAPD, penciclovir or Bis-POM-PMEA. The invention is further directed to a method for treating hepatitis B virus infection comprising administering DAPD in combination or alternation with Bis-POM-PMEA.
Owner:GILEAD SCI INC
Famciclovir sustained-release granules and production method thereof
The invention provides famciclovir sustained-release granules and a production method thereof. The famciclovir sustained-release granules comprise the following components in percentage by weight: 20-30% of famciclovir, 20-40% of adhesives, 2-5% of disintegrants, 2-5% of lubricants and 20-40% of filler. The famciclovir sustained-release granules are low in administration dosage, stable in quality and simple and easily operable in production process, and are suitable for industrial production.
Owner:武汉人福药业有限责任公司
Spherical famciclovir sustained-release capsule preparation method
InactiveCN109288802AIntegrity guaranteedLarge specific surface areaDigestive systemAntiviralsTreatment effectUnit mass
The invention discloses a spherical famciclovir sustained-release capsule preparation method, which comprises: preparation of spherical pellet, preparation of coating layer, and preparation of spherical famciclovir sustained-release capsule. According to the present invention, with the prepared modified starch, the disadvantages that the ordinary corn starch does not have sufficient viscosity, adsorbs the less drug at the unit mass, and cannot achieve the maximized efficacy can be solved; and the spherical famciclovir sustained-release capsule can quickly pass through the intestinal tract, wherein mainly the coating layer is the material formed by the enteric polymer soluble in the intestinal tract, and can slow down the resistance between the drug and the intestinal tract so as to make the drug quickly enter the stomach to provide the treatment effect, the coating layer is slowly dissolved under the action of gastric acid after the drug enters the stomach, and the efficacy of the spherical pellet is slowly released, such that the drug release can be delayed in the human body, and the efficacy can be provided for a long time so as to reduce the drug taking frequency of the patient.
Owner:安徽鼎旺医药有限公司
Drying process for preparing crystalline solid famciclovir
The present invention provides a drying process for preparing crystalline solid famciclovir form I comprising the steps of: i) preparing a wet crystalline form of famciclovir; ii) drying the wet crystalline form of famciclovir at a temperature of below 50° C. until the crystalline form contains less than 15% (wt / wt) wetness; and iii) drying the wet crystalline form of famciclovir at a temperature of above 50° C. until the wet crystalline form of famciclovir contains less than 0.05% (wt / wt) water to obtain crystalline solid famciclovir form I.
Owner:TEVA PHARM USA INC
Pharmacological modulation of telomere length in cancer cells for prevention and treatment of cancer
InactiveUS20090137503A1Particular utilityUncontrolled cell growthBiocideDispersion deliveryTelomeraseCancer cell
Acyclic nucleoside analogs such as acyclovir, ganciclovir, penciclovir and the corresponding pro-drugs, i.e., valacyclovir, valganciclovir and famciclovir, respectively have been identified as inhibitors or antagonists of both telomerase (encoded by TERT) and reverse transcriptase encoded by L-1 (LINE-1) RT, and as useful for treating or preventing cancers induced or mediated by the two enzymes. Method of treating or preventing such cancers in patients involves administration of a therapeutically effective amount of a composition having an inhibitor or antagonist of the reverse transcriptases in cells of the patients. The inhibitor or antagonist blocks lengthening of telomeres in telomerase positive and telomerase negative cells. Methods and kits for detecting pathologically proliferating cells expressing TERT and L1RT are also disclosed.
Owner:ALT SOLUTIONS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





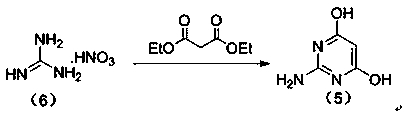








![2-amino-9-(2-substituted ethyl)purines and preparing methods for 9-[4-acetoxy-3-(acetoxymethyl)but-1-yl]-2-aminopurine using the same 2-amino-9-(2-substituted ethyl)purines and preparing methods for 9-[4-acetoxy-3-(acetoxymethyl)but-1-yl]-2-aminopurine using the same](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1a32b8c6-9628-48bc-9304-f2a81748a64e/US07456282-20081125-C00001.png)
![2-amino-9-(2-substituted ethyl)purines and preparing methods for 9-[4-acetoxy-3-(acetoxymethyl)but-1-yl]-2-aminopurine using the same 2-amino-9-(2-substituted ethyl)purines and preparing methods for 9-[4-acetoxy-3-(acetoxymethyl)but-1-yl]-2-aminopurine using the same](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1a32b8c6-9628-48bc-9304-f2a81748a64e/US07456282-20081125-C00002.png)
![2-amino-9-(2-substituted ethyl)purines and preparing methods for 9-[4-acetoxy-3-(acetoxymethyl)but-1-yl]-2-aminopurine using the same 2-amino-9-(2-substituted ethyl)purines and preparing methods for 9-[4-acetoxy-3-(acetoxymethyl)but-1-yl]-2-aminopurine using the same](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1a32b8c6-9628-48bc-9304-f2a81748a64e/US07456282-20081125-C00003.png)